Contrast-enhanced mammography (CEM) is an emerging breast imaging technology that provides recombined contrast-enhanced images of the breast in addition to low-energy images analogous to a 2-dimensional full-field digital mammogram. Because most breast imaging centers do not use CEM at this time, a detailed overview of CEM implementation and performance is presented. Thereafter, the potential use of CEM for supplemental screening is discussed in detail, given the importance of this topic for the future of the CEM community. Diagnostic performance, safety, and cost considerations of CEM for dense breast tissue supplemental screening are discussed.
Key points
- •
Contrast-enhanced mammography (CEM) implementation and performance are discussed in detail to support new use of CEM at breast imaging centers.
- •
CEM may be used for a variety of indications and is recently under study to provide supplemental screening for women with dense breast tissue.
- •
CEM has sensitivity for cancer detection on par with contrast-enhanced breast MR imaging but has associated risks from iodinated contrast administration and radiation exposure.
Introduction
Contrast-enhanced mammography (CEM) remains an emerging technology despite being approved by the Food and Drug Administration (FDA) for clinical use since 2011. Over recent years, the number of published research studies on CEM have rapidly increased and show that CEM has sensitivity on par with MR imaging for breast cancer detection. , However, concerns regarding iodinated contrast reactions, relatively low reimbursement, current lack of commercially available direct biopsy capability, and competition with modalities like MR imaging and tomosynthesis have slowed the clinical adoption of CEM. , In 2020, CEM is considered to be a promising technology but has not yet achieved widespread usage in the breast imaging community.
Most existing publications on CEM are retrospective reviews from single imaging centers. , Larger prospective CEM trials are now on the horizon, such as the Contrast-Enhanced Mammography Imaging Screening Trial (CMIST) and the Rapid Access to Contrast-Enhanced spectral mammography in women recalled from breast cancer screening (RACER) trial. , The results of such prospective trials may determine whether CEM will ultimately achieve widespread use.
This article first presents a detailed description of CEM implementation and performance. Thereafter, use of CEM for supplemental screening is thoroughly discussed given the potential importance of this topic for the CEM community in coming years. Several thorough scientific reviews have recently been published and do not need to be repeated , , ; therefore, only highlights from CEM literature are discussed in this article.
Part 1: contrast-enhanced mammography implementation
Overview
CEM implementation is likely to be straightforward at most breast imaging centers. The physical and personnel requirements necessary for CEM implementation and performance are discussed here.
Physical Requirements for Contrast-Enhanced Mammography Imaging
CEM may be implemented in existing mammography suites as an upgrade to CEM-capable mammography systems. The ability to add CEM to existing equipment without need for additional space allocation is advantageous compared with other supplemental screening options. For CEM-capable systems, implementation requires a software upgrade from the vendor, insertion of a copper filter into the mammography unit, and access to a standard power injector. If an imaging center does not have CEM-capable mammography systems, then such a system would need to be acquired. CEM-capable mammography systems are also able to perform standard mammography, tomosynthesis, and mammographic-guided or tomosynthesis-guided biopsy and localization procedures.
Although hand injection of contrast for CEM has been described in a limited number of publications, a power injector for contrast administration is preferred to obtain a rapid bolus of contrast and a bolus chaser of normal saline. Other physical requirements for CEM include a designated area for intravenous (IV) line insertion, designated space to monitor patients for contrast reactions, and a standard contrast reaction kit and crash cart. A portable point-of-care unit for creatinine testing is most convenient, although laboratory blood draws for creatinine testing also may be used, when indicated.
Personnel Requirements for Contrast-Enhanced Mammography Imaging
At a minimum, a radiologist and mammography technologist trained in CEM imaging are required to successfully perform CEM. Staff members must be adept with IV line placement, iodinated contrast injection, and treatment of iodinated contrast reactions. In the United States, a physician is typically required to be present when IV iodinated contrast is administered to ensure timely treatment for any severe iodinated contrast reactions.
An employee with sufficient training must screen patients for any contraindications to receive iodinated contrast. For this purpose, a radiology nurse can be particularly helpful to coordinate and assess renal function and pregnancy testing before CEM imaging, when applicable. A technologist or breast imaging nurse also may place the IV line, depending on site preference, and monitor patients for contrast reactions before the patient leaves the imaging suite.
Providing education to referring providers may promote success of a new CEM program. Referring providers should understand that CEM allows contrast-enhanced breast imaging for patients who cannot complete MR imaging for any reason, including a direct contraindication (such as a metallic implant that is not MR imaging safe), claustrophobia, patients who are too large to fit within an MR imaging scanner, or imaging expense. In addition, given rising concern regarding the safety of gadolinium contrast used in MR imaging, CEM can be presented as an option for patients and providers who prefer to avoid IV gadolinium.
Part 2: contrast-enhanced mammography performance
The performance of CEM requires attention to ensure patient adequacy for CEM imaging before imaging, strict adherence to image acquisition standards, and patient monitoring following CEM. An example CEM workflow is shown in Fig. 1 .
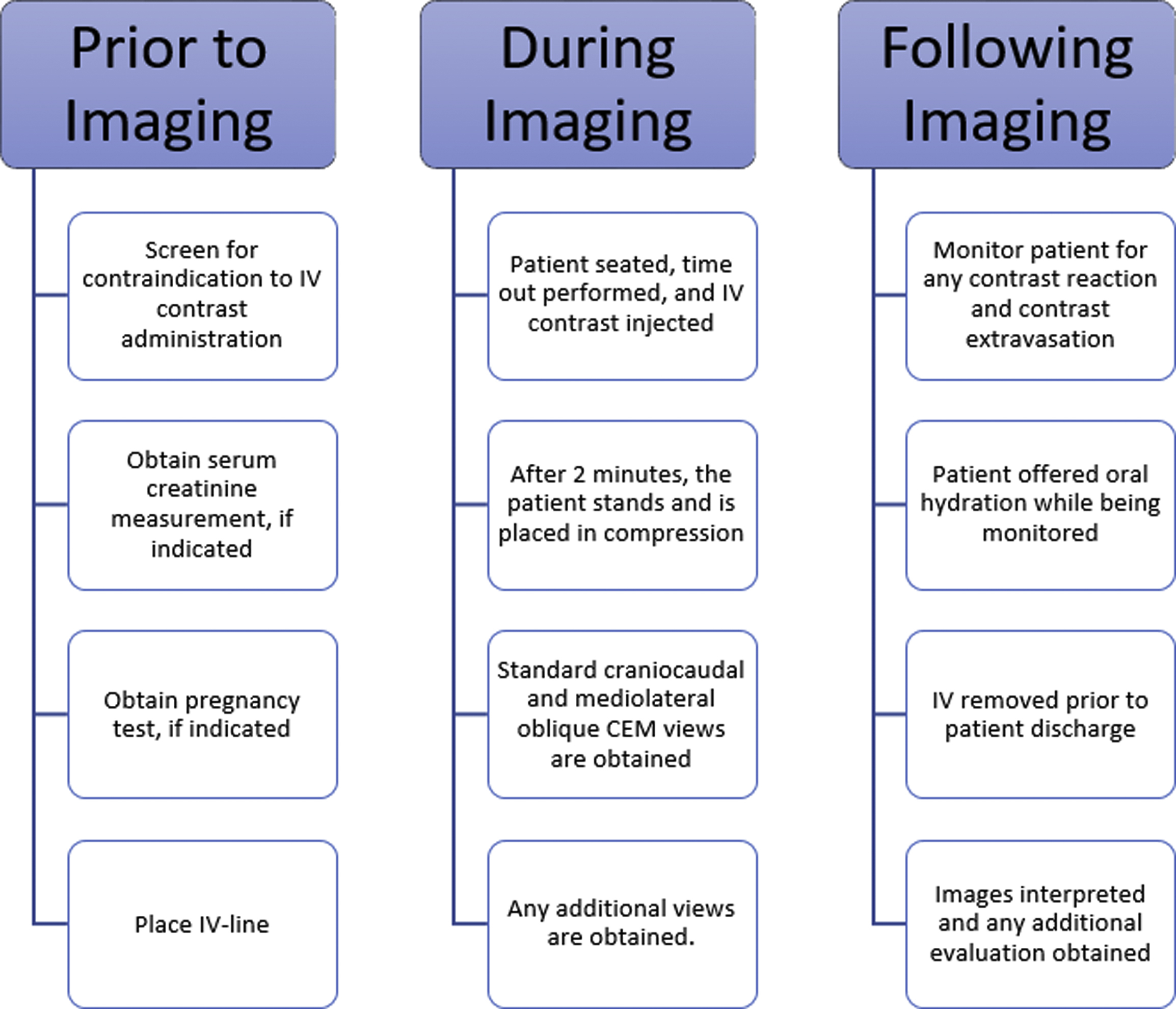
Preparation Before Contrast-Enhanced Mammography Imaging
Before CEM imaging, a patient must be screened for any contraindication to receive IV iodinated contrast. Potential contraindications to receive IV iodinated contrast include glomerular filtration rate less than 30 mL/min per 1.73 m 2 or known allergy to iodinated contrast materials. Other exclusions to CEM imaging may include pregnancy and young patient age. It is not established whether CEM should be timed with a woman’s menstrual cycle, although published literature has described performing CEM between day 5 and day 14 of the menstrual cycle.
In accordance with the American College of Radiology (ACR) Manual on Contrast Media, a serum creatinine measurement should be obtained in patients with 1 or more of the following: age older than 60 years, history of renal disease (including dialysis, kidney transplantation, single kidney, renal cancer, or renal surgery), hypertension requiring medical therapy, diabetes mellitus, and use of metformin or metformin-containing drugs. For these patients, a documented creatinine value obtained within 30 days of CEM is considered sufficient if the patient’s condition is stable since the last result.
Immediately before imaging, an IV must be placed. A 20-gauge needle or larger should be placed in an antecubital vein to accommodate the rapid contrast injection. A robust hand vein may also be used, if necessary. If there is a history of unilateral axillary nodal dissection, the contralateral arm is preferred for IV injection.
The patient should be informed before injection that iodinated contrast administration is frequently associated with a diffuse sensation of warmth, flushing, a metallic taste in the mouth, and urinary incontinence. In addition, the patient should be instructed to notify the technologist if there is pain during or following the injection to alert staff regarding potential contrast extravasation at the injection site.
Image Acquisition
A standard CEM examination comprises 4 low-energy views (a craniocaudal [CC] and mediolateral oblique [MLO] view of each breast) that appear nearly identical to a 2-dimensional (2D) full-field digital mammogram (FFDM). Four contrast-enhanced recombined views in the same projections are also obtained simultaneously. Additional diagnostic views, including spot compression views, also may be obtained as tailored for individual case. All CEM images are taken after the IV administration of iodinated contrast material (such as Iohexol or equivalent, 1.5 mL/kg) that is typically injected at a rate of 3 mL/s followed by an additional 10-mL saline flush.
An example workflow may have an IV placed by a nurse or technologist, after which time the patient is seated in the mammography suite, a time out is performed, the IV line is connected to the power injector, a tight seal between the power injector line and IV tubing is confirmed, and the IV is assessed for patency with a saline flush. Contrast is thereafter injected through the IV, and the patient remains seated as contrast washes into the breast for 2 minutes. During this time, the patient should be monitored for any signs of contrast extravasation or any early contrast reaction. After 2 minutes, the technologist positions the patient and obtains all views within a window of 2 to 6 minutes following contrast injection for a total imaging time of approximately 8 to 10 minutes.
CEM imaging should begin no earlier than 2 minutes after IV contrast administration to allow adequate time for contrast to wash into the breast. The patient’s breast should not be in compression during the initial 2 minutes after contrast injection, as compression will impede blood flow and limit contrast entry into the breast. There is no universal standard as to whether imaging should begin 2 minutes after the start of contrast administration or 2 minutes after the completion of contrast administration. It is possible that imaging at 2 minutes after the completion of contrast administration allows slightly more time for contrast to enter the breast, which could improve the conspicuity of contrast enhancement within breast lesions. Further research is necessary to determine if there is an advantage to either of these approaches.
Several strategies exist to make certain all desired images are obtained within 6 minutes after contrast injection. First, each case should be reviewed in advance to determine whether any views in addition to the standard CC and MLO views of each breast should be obtained. For example, if a lesion is in the far lateral and posterior breast, an exaggerated CC view could be requested in advance. Second, having a more experienced technologist position and obtain images may allow extra time for additional images to be obtained and reduce the likelihood that an image may need to be repeated for technical reasons. Depending on availability, having 2 technologists in the room can be beneficial to allow the first technologist to focus solely on positioning while the other technologist readies and performs contrast injection and image acquisition on the mammography control console. Last, having the radiologist directly in the mammography imaging suite at time of imaging can allow the radiologist to review images as they are obtained and direct the technologist as to whether any views need to be repeated or if any additional views need to be obtained based on review of imaging in real-time. Alternatively, the radiologist could review images in the reading room immediately after they are obtained and direct the technologist(s) regarding any need to obtain additional views.
Contrast-Enhanced Mammography Views
Contrast-enhanced recombined images are obtained through a dual-energy logarithmic subtraction technique that removes breast parenchymal tissue from the image and provides an image that highlights areas of contrast enhancement. The dual-energy technique obtains the low-energy image (26–32 kVp) and then a high-energy image (44–50 kVp) that straddles the 33.2 keV k-edge of iodine. , These images are obtained in rapid succession while the breast is held in the same compression. Low-energy images and recombined postcontrast images are subsequently sent to a picture archiving and communication system for interpretation. The high-energy images are not included for image interpretation but are rather obtained solely to allow for logarithmic subtraction, and neither the low nor high energy depict areas of contrast enhancement. Compared with 2D FFDM and tomosynthesis, CEM derives diagnostic advantage from the removal of breast parenchymal tissue via logarithmic subtraction and the administration of iodinated contrast materials that highlight areas of increased blood flow via tumor-induced angiogenesis. Like contrast-enhanced breast MR imaging, this technique overcomes the masking effect of dense breast tissue on a standard mammogram that facilitates detection of mammographic-occult and tomosynthesis-occult cancers.
A CC and MLO view of each breast should be routinely obtained for a CEM examination, analogous to routinely imaging each breast for a breast MR imaging study. Both CEM and MR imaging demonstrate variable degrees of background parenchymal enhancement and comparison of the intensity and extent of enhancement in each breast is necessary to assess for background enhancement versus pathologic enhancement in each breast.
No current standard exists as to the order of which CEM views should be obtained. However, obtaining images in a consistent pattern may aid the interpretation of a CEM study. For example, if one routinely obtains MLO views of each breast first, followed by CC views, then MLO views will routinely represent an earlier phase of enhancement than CC views, allowing a rough albeit not-as-yet validated assessment of enhancement kinetics within a mass. In such a scenario, if a mass demonstrated robust enhancement on the MLO images obtained first, compared with the CC images obtained later, one could suppose that rapid arterial enhancement with washout may be present. Others have looked at the degree of enhancement on CEM as a predictor of malignancy, with strong enhancement being more predictive of malignant lesions. ,
Note that formal kinetic assessment is theoretically possible with CEM but is not performed due to the multiple radiation exposures that would result from obtaining sequential images in the same projection over time. Obtaining views of each breast in the same projection before proceeding with another projection (for example an MLO view of each breast before obtaining CC views) will maximally ensure that a corresponding pair of images is available for each breast if imaging is terminated early for any reason. An example workflow for image acquisition is shown in Fig. 2 . If the degree of contrast enhancement in the breast appears weaker than expected on initial images (such as when performing extent of disease evaluation for a known malignancy) the first view(s) could be repeated at the end of the study to assess for improved depiction of enhancement on more delayed imaging.

A combination mode wherein tomosynthesis and CEM images are obtained simultaneously in the same breast compression may be performed. In such a scenario, approximately 2 minutes after IV contrast injection, a tomosynthesis image set may first be obtained, followed by the low-energy and high-energy images of CEM, thereby providing tomosynthesis, low-energy, and subtracted images coregistered from the same compression for direct comparison and review. Imaging that provides 3D assessment of contrast enhancement, or that overlies contrast-enhanced images on tomosynthesis slices, is not currently available. One must be cognizant of the added radiation to the patient from performing tomosynthesis in addition to CEM, as this will result in 3 separate exposures to ionizing radiation per view obtained. In a 2017 phantom study, the average glandular dose per view at a mean phantom breast thickness of 63 mm was 3.0 mGy for CEM compared with 2.1 mGy for 2D FFDM and 2.5 mGy for tomosynthesis. Therefore, the addition of simultaneous tomosynthesis to a CEM study may increase the average glandular dose by approximately 80% (5.5 mGy compared with 3.0 mGy).
After Contrast-Enhanced Mammography Imaging
After imaging, a patient should be monitored for any contrast reaction or extravasation. Duration of monitoring may vary depending on site preference but should be at least 15 minutes after imaging. The patient should be given instructions regarding subsequent self-monitoring for any contrast reaction and be given appropriate follow-up instructions should this manifest. In addition, the patient may be given fluids to hydrate after imaging. The IV line should remain in place until shortly before the patient is discharged because IV access may be needed if a severe contrast reaction develops.
Part 3: contrast-enhanced mammography interpretation
CEM images may be interpreted by the radiologist while the patient is still present, facilitating additional imaging evaluation when necessary, or images may be interpreted after the patient has left the imaging suite. At present, most CEM examinations are performed for diagnostic evaluation rather than screening, and immediate reads may be preferred in that setting. , However, if CEM were used for high-risk screening, standard CC and MLO views of each breast could be obtained by the technologist(s) and either immediate or delayed reads could be performed.
CEM interpretation may have a low learning curve for breast imagers because CEM blends the interpretation standards and reporting lexicon of mammography and contrast-enhanced breast MR imaging. , A CEM report should contain a description of breast density based on the low-energy (2D FFDM-like) images according to the current recommendations of the ACR Breast Imaging-Reporting and Data System (BI-RADS) Atlas. , Like breast MR imaging, the degree of background parenchymal enhancement based off the recombined postcontrast images should be reported as minimal, mild, moderate, or marked. An example CEM reporting template is shown in Fig. 3 .

Low-energy CEM images have been shown to be noninferior to 2D FFDM views for mammographic interpretation. A CEM report should describe typical mammographic findings based on the low-energy images in terms of masses, microcalcifications, architectural distortion, asymmetries, and so forth, according to standard BI-RADS recommendations for mammographic imaging. If concurrent tomosynthesis imaging was performed, any tomosynthesis findings should be reported along with low-energy CEM imaging findings.
Recombined postcontrast images may be reported following the MR imaging reporting lexicon from BI-RADS. , , , Evaluation of the recombined postcontrast images includes assessment of abnormal areas of enhancement in terms of foci, masses, and nonmass enhancement as in breast MR imaging. When appropriate, size measurements may be reported separately for the low-energy and recombined postcontrast images, or in unison if there is agreement. Prior studies have shown that the size measurements of a lesion on CEM are comparable to those of MR imaging. ,
Finally, a BI-RADS assessment category and appropriate follow-up recommendations should be reported, according to the standard BI-RADS lexicon. A site also may choose to include details regarding type and amount of IV contrast and the patient’s creatinine and glomerular filtration rate values in the clinical report.
Part 4: further management of contrast-enhanced mammography imaging findings
Further Evaluation of Suspicious Contrast-Enhanced Mammography Lesions
Any new suspicious finding identified on CEM images should be assessed with recent or follow-up diagnostic mammography and ultrasound to establish benignity, or else identify a target for follow-up or biopsy. For most cases, a corresponding target will be evident on additional diagnostic views and/or CEM-directed ultrasound. A potential advantage of CEM compared with MR imaging is that the location of CEM enhancement on standard CC and MLO views is easier to correlate on additional mammogram and ultrasound view compared with MR imaging that is not obtained in mammographic imaging planes. When a correlating lesion is biopsied, the clip location on postbiopsy mammography may be directly compared with the location of CEM enhancement to assess for appropriate tissue sampling. Determining exact positional concordance between postbiopsy mammogram and MR imaging is less accurate.
Depicting the location and extent of abnormal contrast enhancement on CEM also allows direct comparison with mammography at time of mammographic localization for presurgical bracketing. Extrapolating the extent of abnormal enhancement seen on MR imaging to a mammogram for presurgical localization or bracketing is not as easy given that MR imaging has different positioning compared with mammography.
Suspicious CEM enhancement should be correlated with diagnostic mammography, tomosynthesis, and/or targeted ultrasound to identify a biopsy target. If no biopsy target is seen on these modalities, contrast-enhanced breast MR imaging would then be necessary for further evaluation and to guide subsequent biopsy, if indicated.
Biopsy of Suspicious Contrast-Enhanced Mammography Lesions
Current CEM systems are not capable of biopsy with direct CEM guidance, although a CEM biopsy system has been announced by at least 1 vendor, pending FDA 510k approval. Several work-around options exist whereby the CEM-detected lesions may ultimately be sampled. The first option is to biopsy a correlating lesion using ultrasound, tomosynthesis, or stereotactic biopsy. In certain cases, finding a tomosynthesis correlate may be aided by obtaining coregistered tomosynthesis images at time of CEM imaging. If no correlating lesion is identified on mammogram or ultrasound, MR imaging should be performed to identify a corresponding lesion for MR imaging–guided biopsy. If no lesion is seen on MR imaging, then short-interval CEM follow-up rather than biopsy may be indicated.
A method has been described whereby a lesion may first be localized under direct CEM guidance for placement of a biopsy clip, radioactive seed, or other localization device at the area of abnormal CEM enhancement; subsequently, biopsy targeting the clip or seed may be performed using tomosynthesis or stereotactic biopsy versus surgical excisional biopsy.
Part 5: potential use of contrast-enhanced mammography for supplemental screening
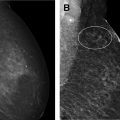
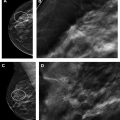
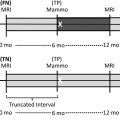
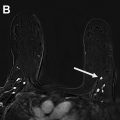
Multiple comprehensive CEM literature reviews have recently been published. , , , To date, CEM has been evaluated for many uses including presurgical evaluation for extent of disease and staging of known malignancy , , ( Fig. 4 ), neoadjuvant therapy response monitoring, , abnormal findings on screening mammography/complicated mammography troubleshooting, imaging of patients with an MR imaging contraindication, symptomatic breast evaluation, , , , evaluation of indeterminate mammographic calcifications, , , evaluation of mammographic architectural distortion ( Fig. 5 ), mammographic imaging for women with dense breast tissue and/or high-risk supplemental screening , , ( Fig. 6 ), to reduce biopsy rate for low to moderate suspicion soft tissue lesions, and postoperative breast cancer surveillance.