Fig. 1
Ductal carcinoma in situ (DCIS): malignant cell growth resulting in a distension of the terminal-duct lobular unit (TDLU). Various other benign proliferative or fibrocystic changes develop along a distinct genetic and morphological pathway and can also result in a distension of the TDLU. Associated calcifications within the TDLU develop in DCIS, fibrocystic changes, sclerosing adenosis, and other forms of adenosis. Expanded TDLUs can be depicted by mammography, ultrasound, or magnetic resonance mammo – graphy. Corresponding diagnostic criteria are listed in the text (for color reproduction see p 318)
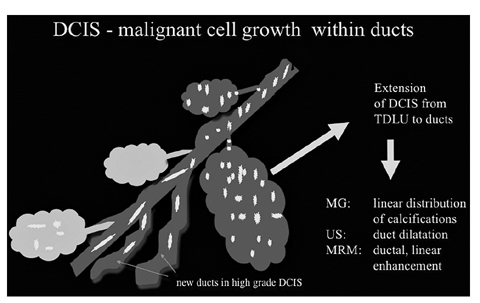
Fig. 2
Ductal carcinoma in situ (DCIS): malignant extension of DCIS into the ducts and other terminal-duct lobular units (TDLUs). New ducts can be formed in high-grade DCIS. Corresponding diagnostic criteria are given and focus on the linear extension or dilatation of ducts in all imaging modalities (for color reproduction see p 318)
The echogenicity of fat is the reference for comparing other anatomical structures within breast US [1, 2]:
Isoechoic echogenicity is found in fat, epithelium, loose periductal and intralobular fibrous tissue and some TDLUs
Hyperechoic echogenicity is found in skin, Cooper’s ligaments, stromal fibrous tissue (interlobular) and some TDLUs
Hypoechoic echogenicity is found in nipple, blood vessels and some TDLUs
Anechoic echogenicity is found in dilated TDLUs (cysts), ducts and lymphatics
Physics and Equipment
US of the breast provides physical information about the impedance of tissue interfaces that influence US transmission and reflection across the breast. The different physical base of US, X-ray mammography and MRI of the breast explains the independent and complementary diagnostic information given by each modality. The most relevant advances made in recent years are due to highfrequency US transducer equipment using frequencies between 7 and 18 MHz. Scanning with 15 MHz in comparison with 7.5 MHz results in a lateral (0.4 mm) and axial (0.2 mm) spatial resolution that is twice as high as the spatial resolution at 7.5 MHz. On the other hand, penetration depth is reduced to half. Compounding and harmonic imaging improves contrast resolution and reduces speckle artefacts. The high spatial and contrast resolution of modern breast US equipment has expanded the detection and conspicuity of subtle lesions the size of expanded TDLUs such as DCIS and microinvasive lesions. Color Doppler techniques detect and characterize blood flow within lesions, and this allows discrimination between solid nodules and complicated cysts. Three-dimensional (3D) diagnostic imaging of the breast includes multidimensional reformations, reconstructions and tomographic US. The additional diagnostic information of 3D US focuses on demonstrating suspicious radial retractions around a tumor in the coronal plane, which is unique to this technique. Elastography reflects strain properties of lesions. Malignant nodules are generally less compressible than benign tissue. Strain, shear wave and semistatic elastography are the actual techniques to assess tissue stiffness. Elastography can downstage BI-RADS® 3 lesions independent of the applied technique. The future role of elastography continues to be evaluated. New horizons in high-end US technology encompass miniaturized and portable US systems, and automated whole breast US, and imaging fusion of US information with digital mammography, tomosynthesis, contrast enhanced dual energy mammography, MRI or positron emission tomo — graphy [3, 4].
Indications for Breast Ultrasound
A list of updated recommendations pertaining to indications is given in Box 1 (modified according to [3]). US is the first-line imaging technique for women <40 years presenting with symptoms or clinical signs. In the presence of a suspicious lesion, US is the method of choice to guide core biopsy in order to harvest tissue. US-guided VAB is used increasingly to diagnose intraductal lesions, small architectural distortions and borderline lesions; to complete preoperative staging in patients with extensive ductal component; and for therapeutic excision. Stereotactic- guided VAB is the method of choice to sample screen-detected microcalcifications and architectural distortions not seen on US. In the dense breast, the combination of US and screening mammography improves cancer detection considerably compared with mammo – graphy alone, but with an increase in biopsy rate. The additional diagnostic yield of US after negative mammo – graphy is 3.2:1,000 women with dense breasts. Intraoperative surgeon-performed US focuses on accurately defining the resection segment or sector and the margin analysis of the resection specimen. MRI is useful preoperatively to assess the extent of ipsilateral disease and exclude contralateral breast cancer, particularly for women at increased risk of mammographically occult disease. Second-look US can detect up to 50% of magnetic-resonance- enhancing cancers with negative mammography [5–8].
Box 1
Updated indications for high-resolution US
Differentiation of cysts and solid tumors | |
Differentiation between solid, benign and malignant lesions | |
Characterization of palpable abnormalities | |
Assessment of mammographic screening abnormalities | |
Dense breasts showing with reduced mammographic sensitivity | |
Diagnosis and follow-up of women with benign breast disease or risk lesions | |
Women, during pregnancy or lactation | |
Significant nipple discharge | |
Under hormonal replacement therapy | |
Inflamed breast and abscesses formation | |
Extended screening for high-risk patients | |
Second look after magnetic resonance mammography | |
Guidance of interventional procedures, such as fine needle aspiration, core biopsy, diagnostic and therapeutic vacuum biopsy and preoperative tumor localization, axillary lymph node biopsy | |
Preoperative staging of lesion size, skin and nipple distance for planning breast conservative surgery, mastectomy or oncoplastic reconstruction with implants, assessment of multifocality, multicentricity, intraductal extension, lymph node changes and contralateral lesions | |
Preoperative staging and follow-up under neoadjuvant chemotherapy | |
Surveillance after breast-conservation therapy | |
Silicone implants |
Indications for Breast Ultrasound
A list of updated recommendations pertaining to indications is given in Box 1 (modified according to [3]). US is the first-line imaging technique for women <40 years presenting with symptoms or clinical signs. In the presence of a suspicious lesion, US is the method of choice to guide core biopsy in order to harvest tissue. US-guided VAB is used increasingly to diagnose intraductal lesions, small architectural distortions and borderline lesions; to complete preoperative staging in patients with extensive ductal component; and for therapeutic excision. Stereotactic- guided VAB is the method of choice to sample screen-detected microcalcifications and architectural distortions not seen on US. In the dense breast, the combination of US and screening mammography improves cancer detection considerably compared with mammo – graphy alone, but with an increase in biopsy rate. The additional diagnostic yield of US after negative mammo – graphy is 3.2:1,000 women with dense breasts. Intraoperative surgeon-performed US focuses on accurately defining the resection segment or sector and the margin analysis of the resection specimen. MRI is useful preoperatively to assess the extent of ipsilateral disease and exclude contralateral breast cancer, particularly for women at increased risk of mammographically occult disease. Second-look US can detect up to 50% of magnetic-resonance- enhancing cancers with negative mammography [5–8].
Examination Technique
The International Breast Ultrasound School (IBUS) and American College of Radiology (ACR) guidelines for breast US examination advise a systematic, comprehensive and reproducible examination technique, followed by documentation, description, reporting, classification and recommendation. The examination starts with proper positioning of the patient in a supine or anterior oblique position depending on the breast volume, with elevation of the ipsilateral arm. Positioning should result in a maximum flattening of the breast portion being examined. Automated tissue optimization, focal zone and field of view settings should be optimized before scanning with the transducer perpendicular to skin. A minimum of two scan planes is recommended in whole breast US. Image analysis of a detected lesion or pseudolesion requires rotation of the transducer over the entire lesion using changing compression intensities and angulations. Radial imaging of adjacent ducts is mandatory to assess ductal extensions. BI-RADS® descriptors and further criteria of additional elastography, 3D tissue criteria, vascularization and associated lymph node morphology characterise a stateof- the-art lesions assessment by US. The o’clock position and distances to skin and nipple describe the exact localization of a lesion within the volume of the breast. Indication of palpability and imaging correlation to other modalities complete the documentation [9–12].
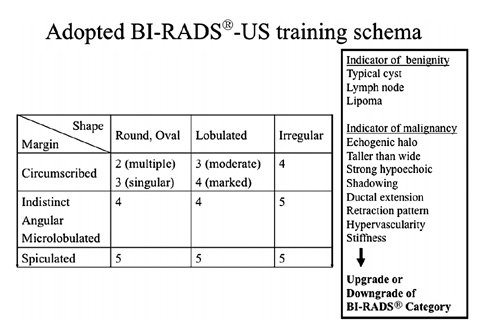
Concepts of Interpretation Based on Ultrasound BI-RADS® Descriptors
The categorization of a mass finding in all modalities relates to a 3D macropathological tissue lesion. The pathology defines lesion shape, margin and texture. These features have already been described individually for the varying modalities. A uniform wording of the major diagnostic criteria for all modalities would be logical. The BI-RADS® concept took a first step in this direction and was designed primarily as a mammographic language with a clear, defined terminology. In 2003, the ACR published the Breast Imaging Atlas, which is a BI-RADS® lexicon for mammography, US and MRI. The US chapters were originally arranged under the chair of Ellen B. Mendelson [11], and the descriptors or diagnostic criteria are presented with increasing probability of malignancy. Descriptors of a mass include shape, orientation, margin, boundary, echo pattern, posterior acoustic feature and characteristics of surrounding tissue, as well as associated distinguishing findings. The combination of several descriptors predicts malignancy better than one single descriptor. However, the reader should use further explanatory elements in the guidance chapters of the atlas, such as clinical context conditions, tumor biology and epidemiological prevalence, to cover the complex field of breast lesions. Assumptions regarding the expected prevalence and individual risk for cancer in a patient drive the intuitive recommendation for or against a biopsy and influence the choice of a final BI-RADS® assessment category. In other words, the threshold for performing a biopsy is lower for a probably benign-looking lesion compared with a screening setting if advanced age, large lesion, palpability or individual high-risk situation concern the reader. BI-RADS® categories 3–5 imply a defined probability of malignancy for each category. For BI-RADS® 3, these probabilities are <2%, for BIRADS R 4 between 3% and 94%, and for BI-RADS® 5 ≥95%. Most European US societies have adopted or modified the ACR BI-RADS® US guidelines. In addition to the 2003 US descriptors, various features have been suggested, such as elastic compressibility, movability, 3D criteria, detailed lymph node morphology and others. Further prospective multicenter studies are needed to validate the complementary diagnostic importance of such associated features as an adjunct to the basic characteristics of a lesion [12, 13]. Recently, several authors disclosed that interobserver agreement with the new BIRADS ® terminology is good, and validated the lexicon in retrospect following landmark studies in the 1990s. Only fair agreement exists in most studies for margin evaluation. Further, a trend towards lower concordance was noted for evaluating small masses. Classification into sub — divisions 4a, 4b and 4c was more or less reproducible. Despite its limitations, most authors agree that stratification predicting the likelihood of malignancy could be useful for decision making and communication with patients, and between researchers, physicians and physicians of different specialties [14–17]. The updated second edition of BI-RADS® US (2013) will re-emphasize the importance of basic features, such as mass shape, margin and orientation on one hand, and associated findings as an adjunct on the other. The amended chapters cover expanded general issues, detailed lexicon images and US descriptors, reporting system and guidance. Fig. 3 presents a training schema for beginners in the field of breast diagnostics that can be used to learn standardized BIRADS ® US reading of larger masses. This schema has no scientific proof for use in daily work-up. Box 2 highlights some underlying intrinsic and extrinsic concepts of BIRADS ® US assessment categories that must be considered in daily work.
Concepts of Interpretation and Clinical Decision Making
The US characterization of a lesion in the daily routine follows a reproducible diagnostic algorithm and should involve fundamental US and all advanced applications of the used US system, preferably on a one-click basis.
First, the reader must define whether or not the lesion resembles a typical benign finding, such as cyst, lipoma, lymph node or previously known scar or fibroadenoma (Fig. 3). Complicated cysts with internal debris are challenging. When the debris is mobile or a fluid-debris level is seen, complicated cysts can be dismissed as benign findings, i.e. BI-RADS® US category 2 [11, 18].
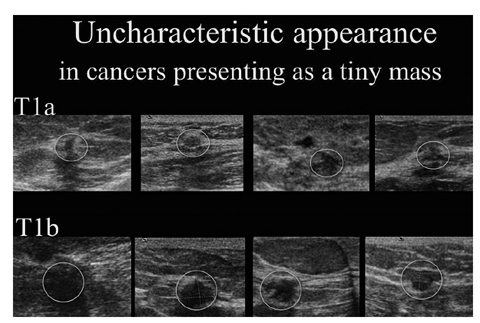

Fig. 3
Uncharacteristic appearance of small cancers stages T1a and T1b. All these cancers have been detected by screening mammogram and correlated to ultrasound secondarily during assessment (courtesy of Screening Centre Southwest Lower Saxony; Praxis Drewes and Partners). Several small benign lesions resemble the presentation of small cancers (for color reproduction see p 319)
Second, a typical oval-shaped, hypoechoic lesion with circumscribed margins and horizontal orientation in young women is most likely a fibroadenoma (Fig. 4). Short-term follow-up can be used. Several studies concluded that short-term follow-up of such BI-RADS® US category 3 lesions is associated with a cancer rate <2% [19–21]. Being older than 45 years, palpability or any preselection that enriched cancer cases in the collective is associated with cancer rates >2%. In a recent study, 0.8% of 4,000 women with lesions that were initially classified as probably benign proved to be malignant at follow-up. The most frequent reason for a false-negative assessment on US was failure to recognize suspicious margin characteristics (28 of 32 malignancies, 87.5%). Malignancy was more frequent in palpable (2.4%, 21 of 859) than nonpalpable lesions (0.4%, 11 of 3,141) [22]. As an isolated finding, homogeneous complicated cysts and clustered microcysts can be classified as probably benign, particularly if the lesion is new or rather small or deep, i.e., diagnostic uncertainty exists [18].
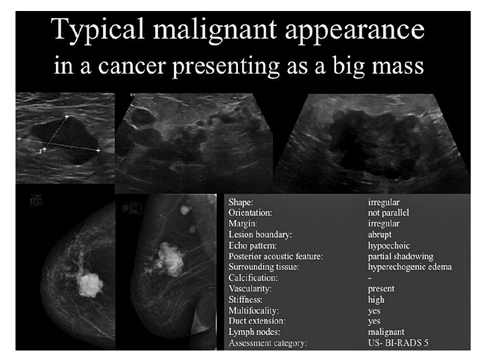

Fig. 4
Typical malignant appearance of ductal invasive cancer presenting as a lump in a 70-year-old patient. Mammography and ultrasound present the tumorous irregular mass, branching pattern of ductal extension, multifocal lesions, and axillary lymph node metastasis showing an expanded cortex. Description is given for the biggest mass following ultrasound Breast Imaging Reporting and Data System
Third, detailed analysis of US morphology, vascularity and elasticity of a lesion should disclose any suspicious basic descriptor or suspicious associated finding. The presence of suspicious descriptors results in a BIRADS® US category 4 or 5 depending on the total number and character of these descriptors. A biopsy is recommended in these cases and also in benign-looking lesions that significantly increase in size during follow-up (Fig. 5) [11a].