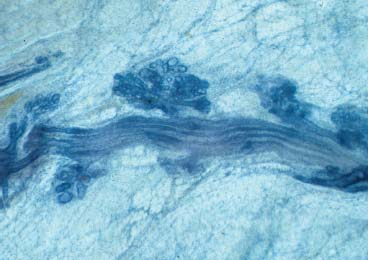
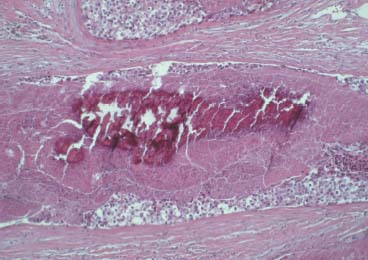
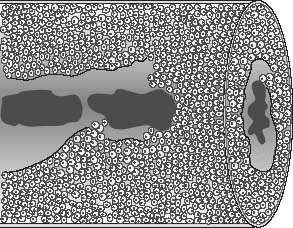
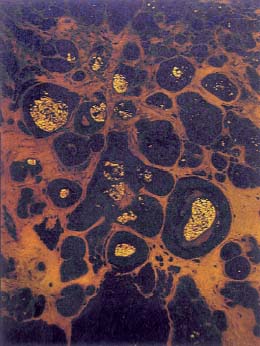
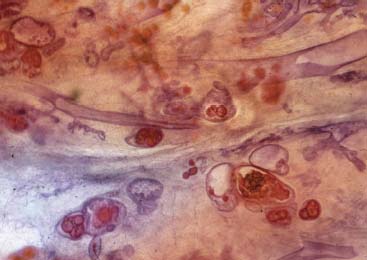
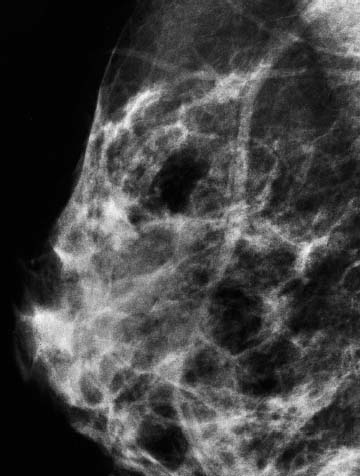
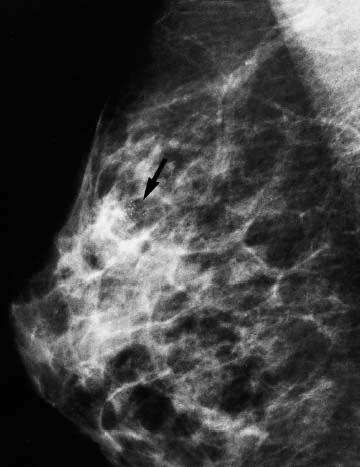
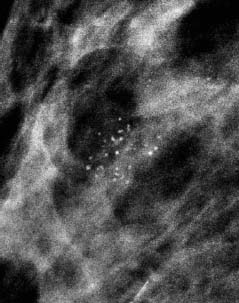
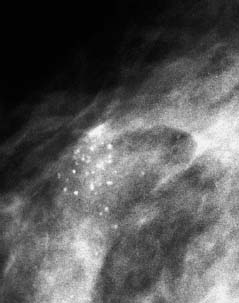
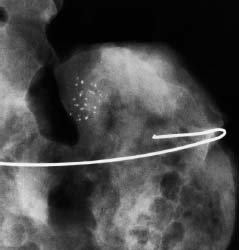
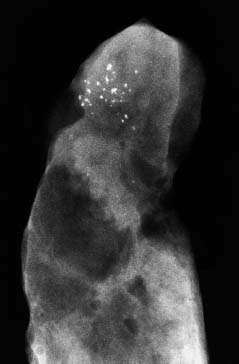
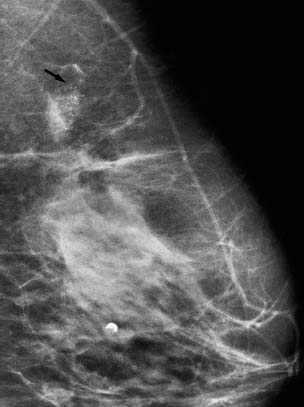
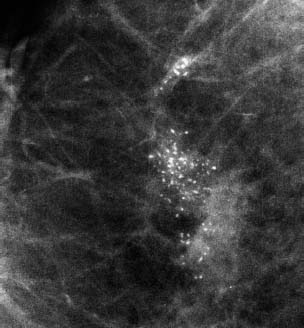
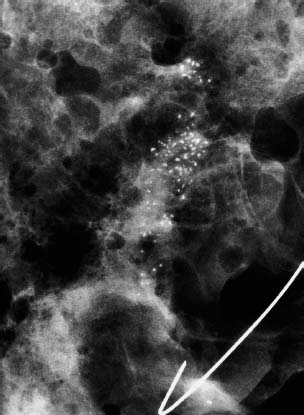
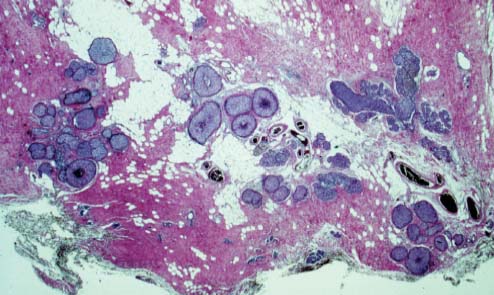
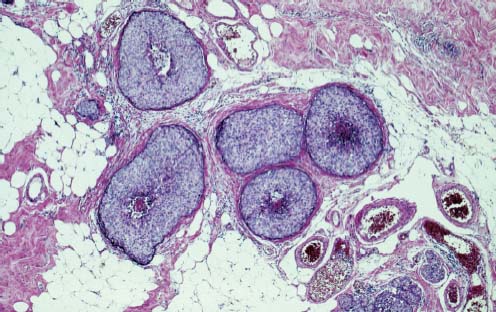
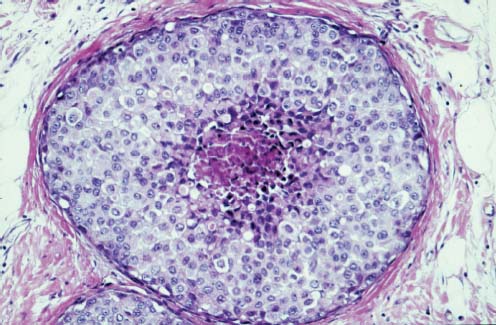
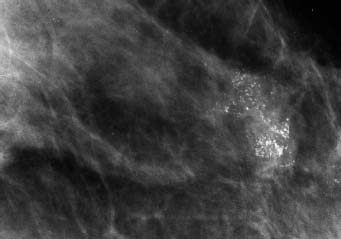
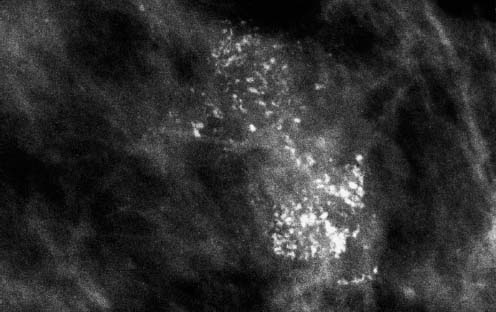
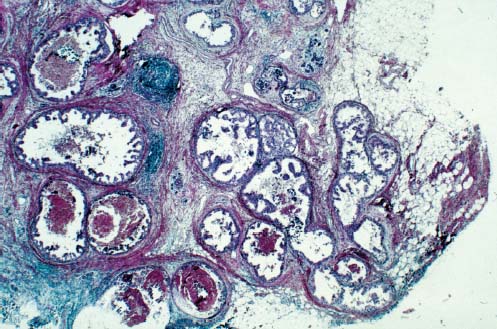
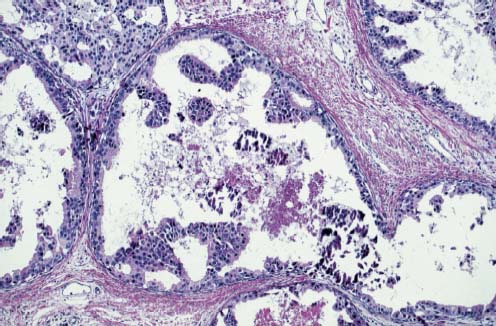
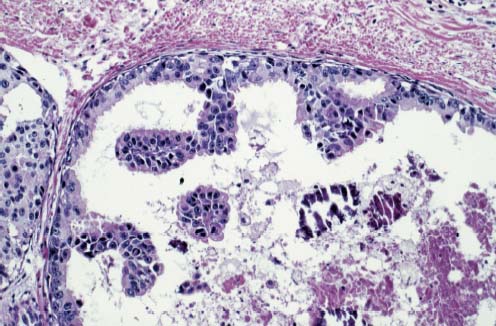
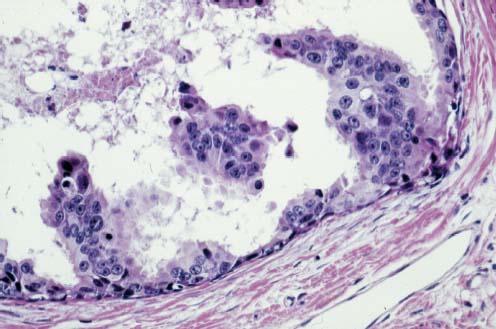
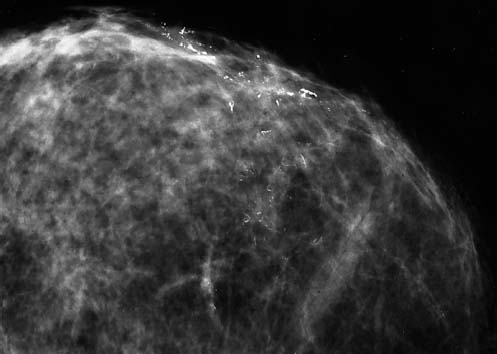
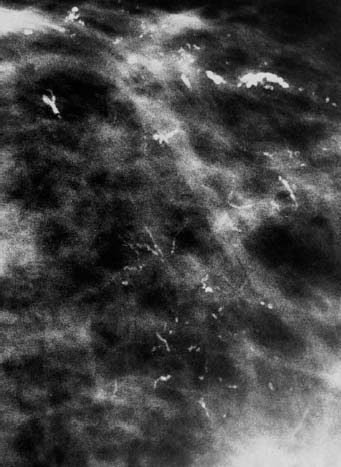
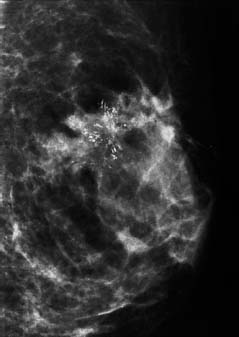
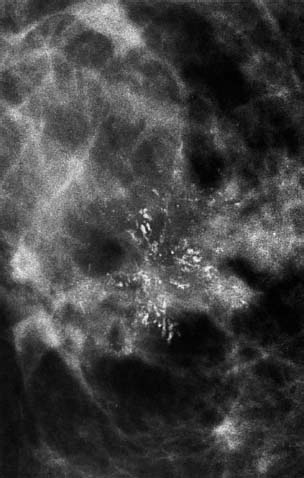
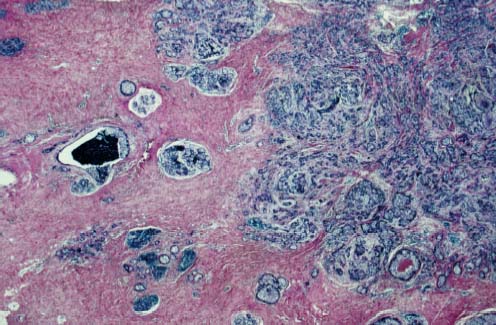
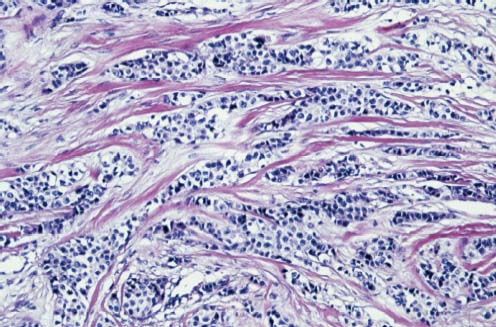
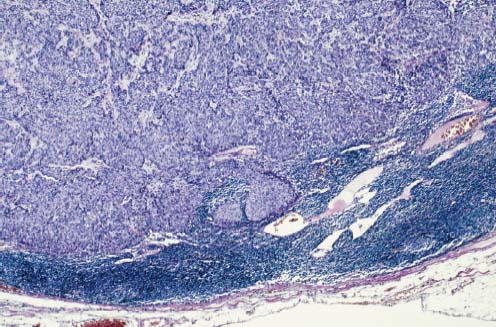
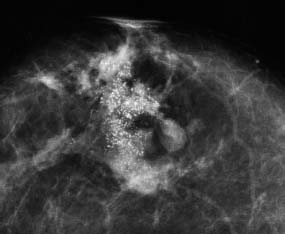
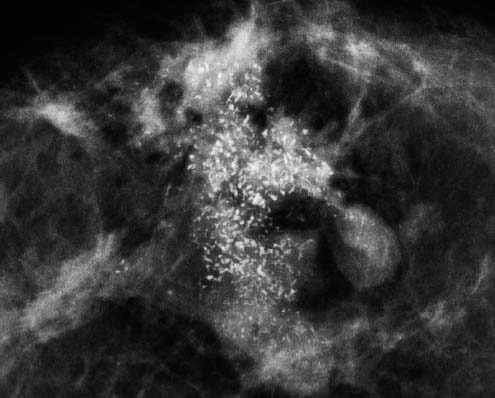
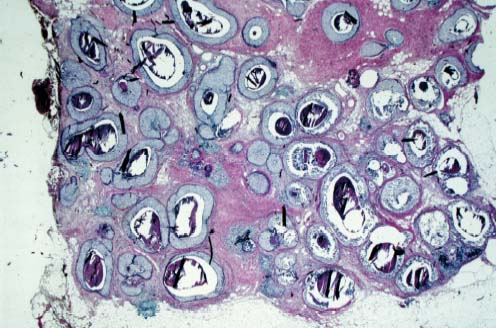
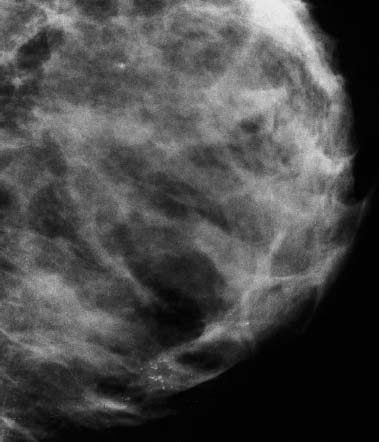
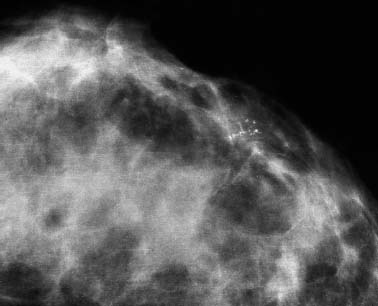
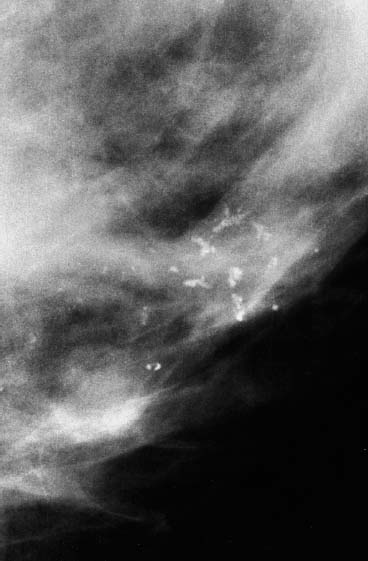
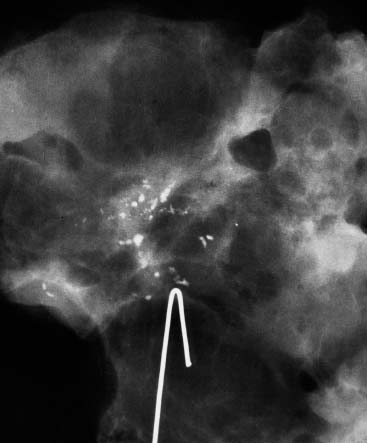
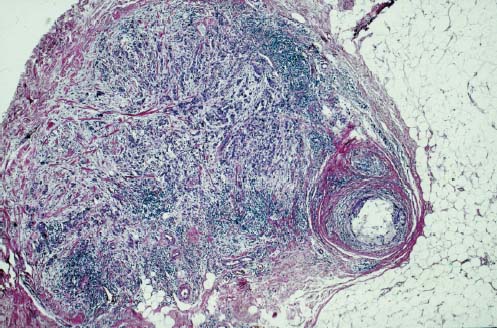
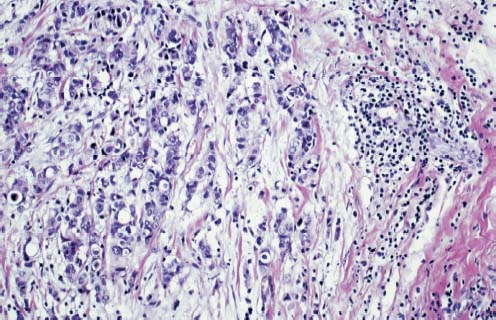
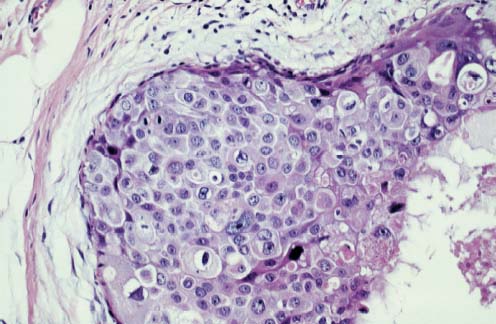
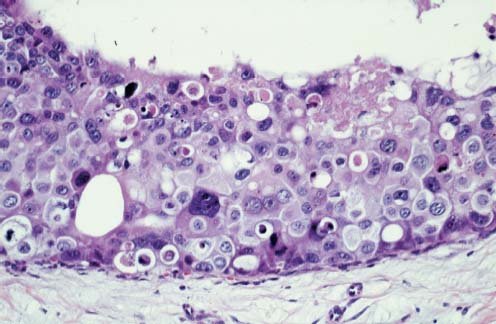
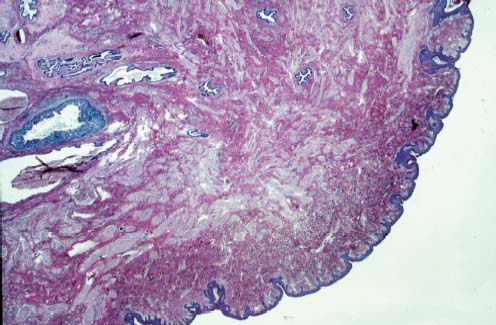
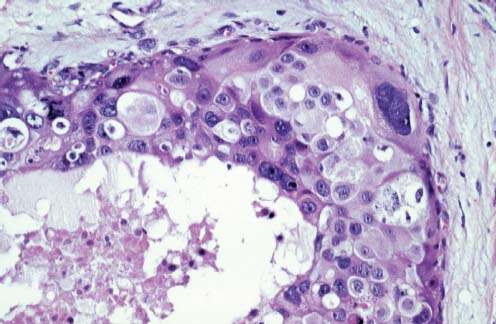
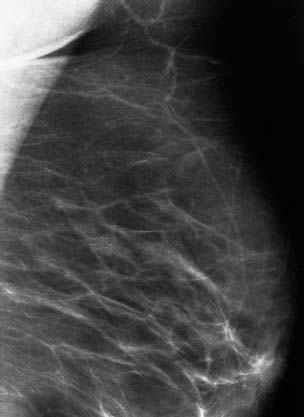
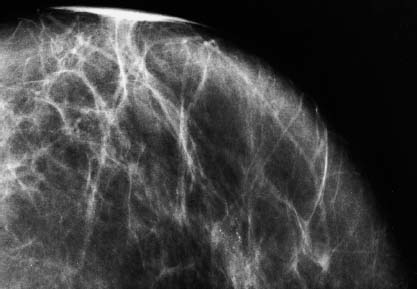
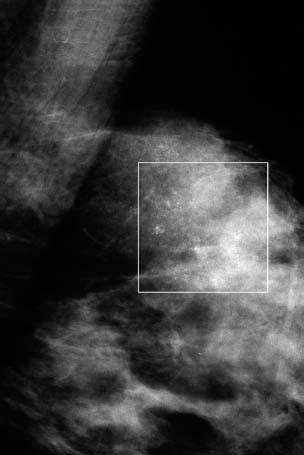
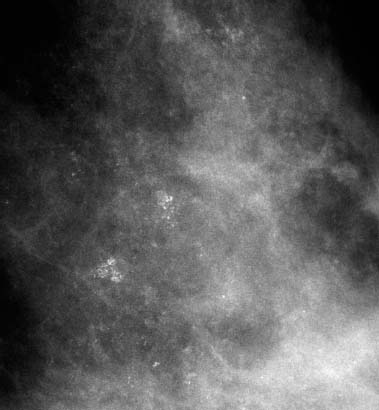
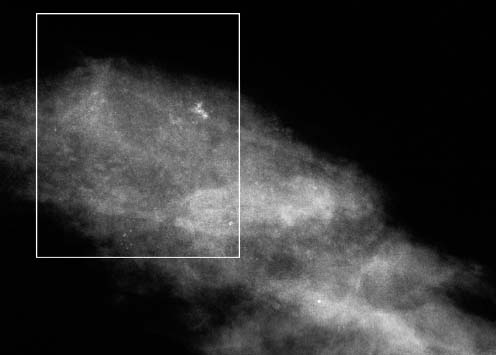
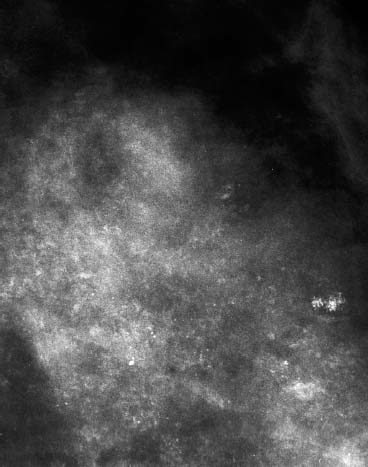
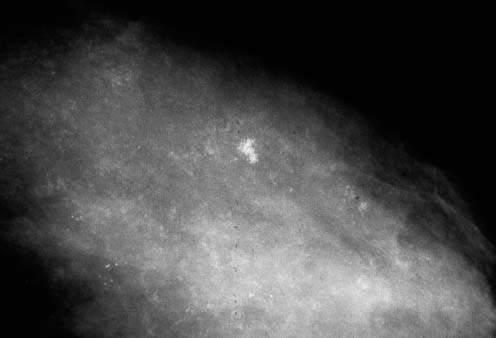
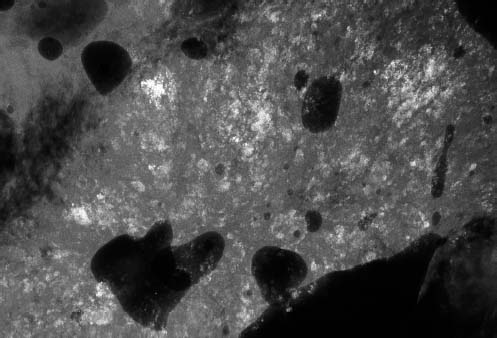
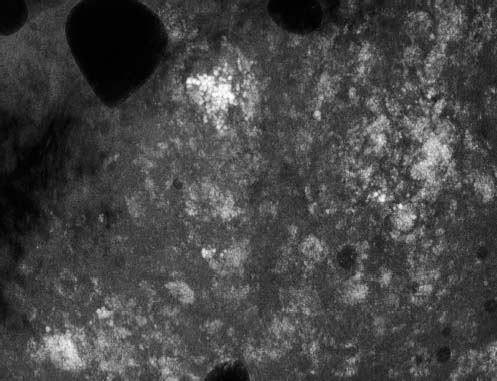
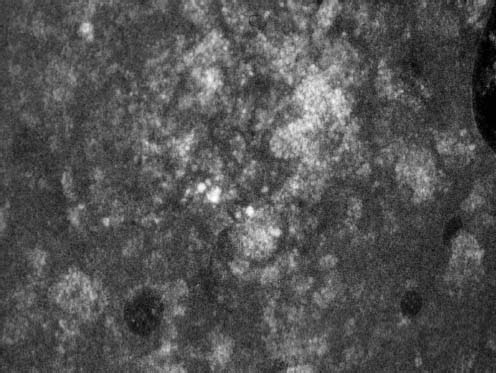
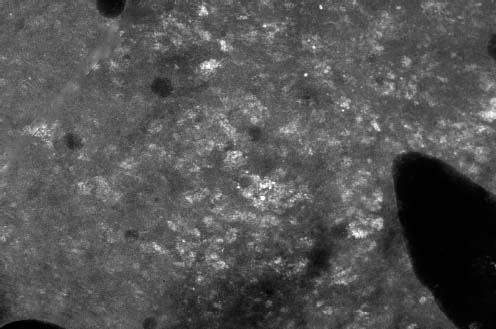
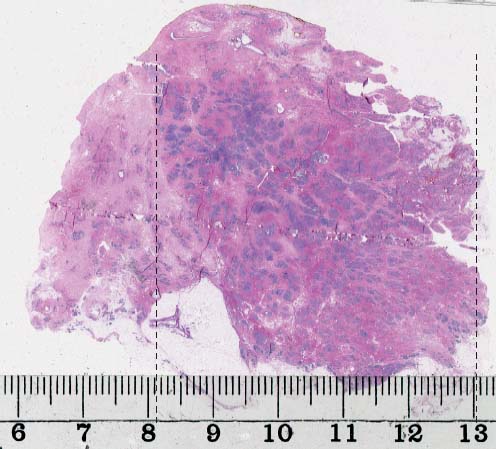
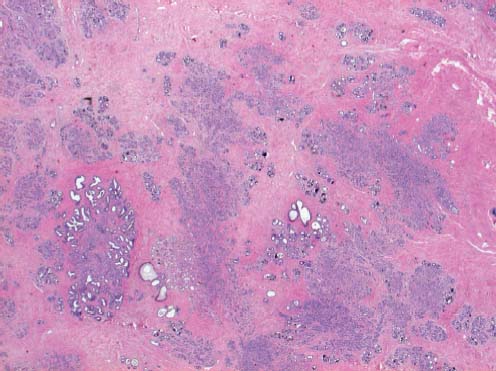
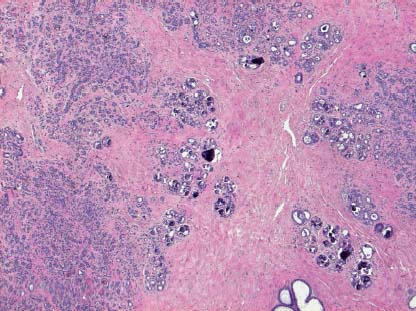
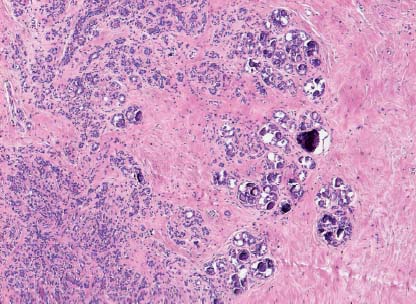
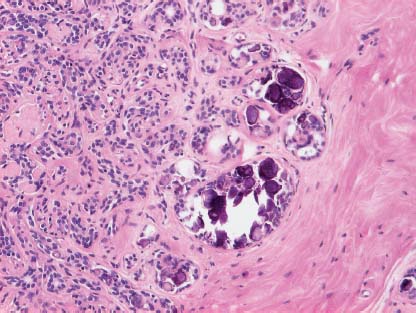
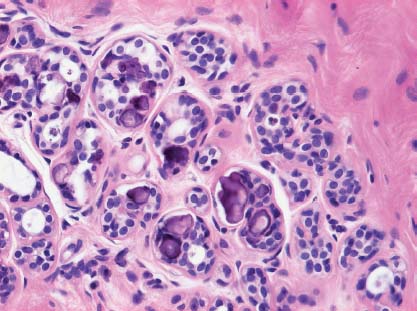
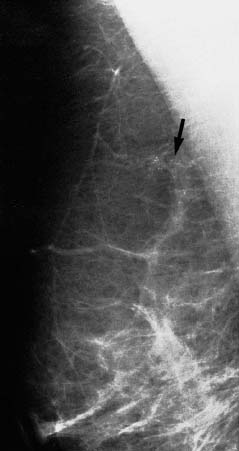
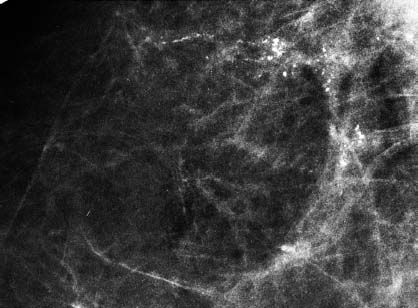
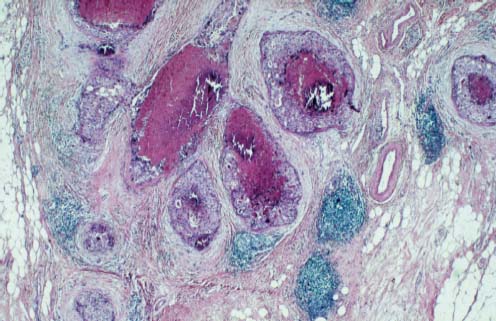
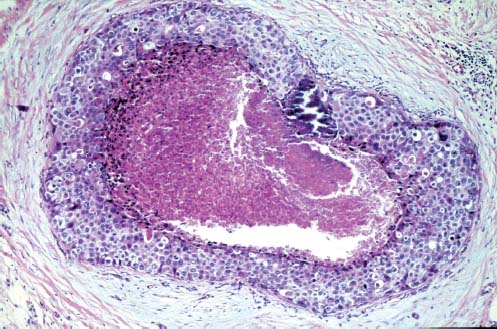
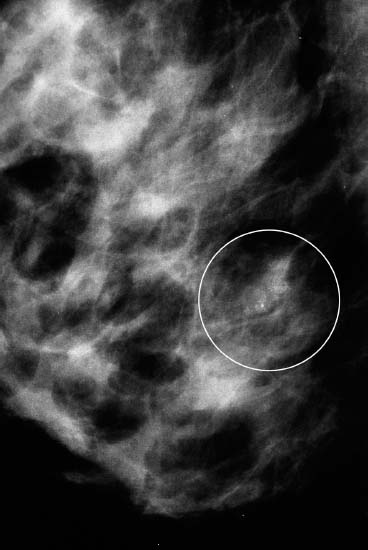
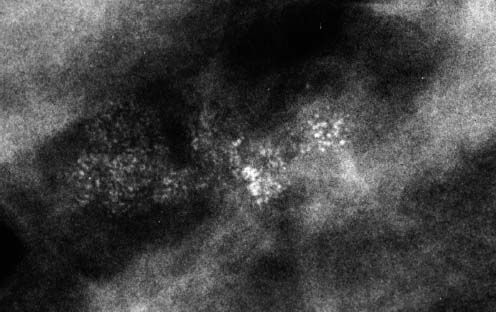
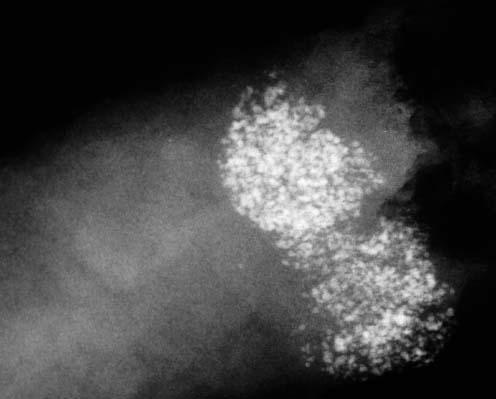
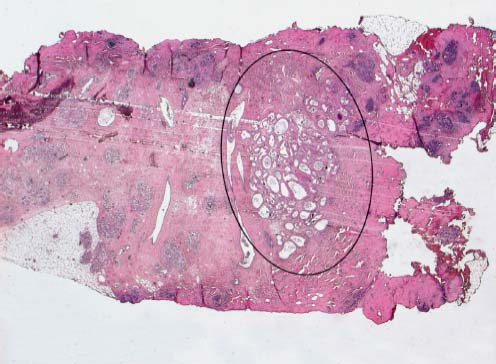
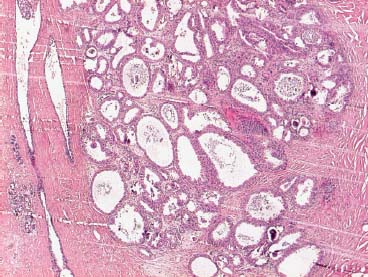
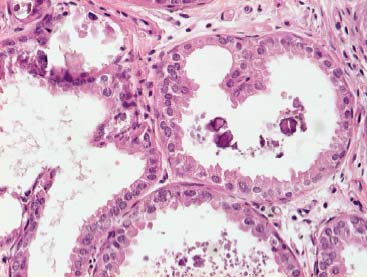
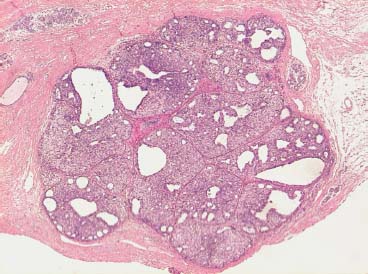
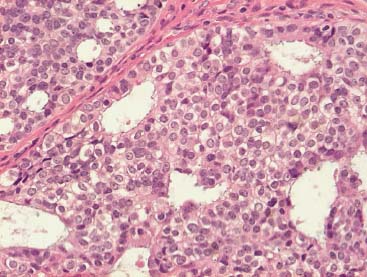
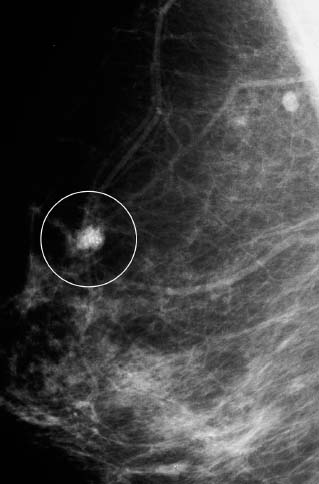
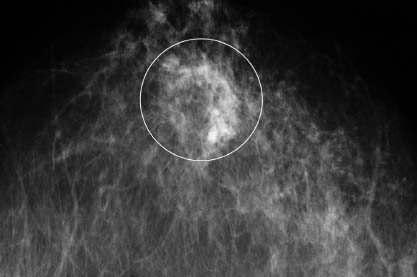
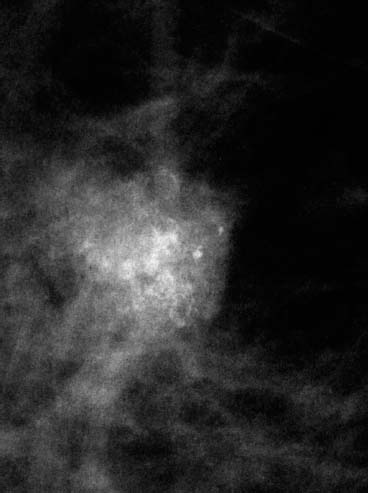
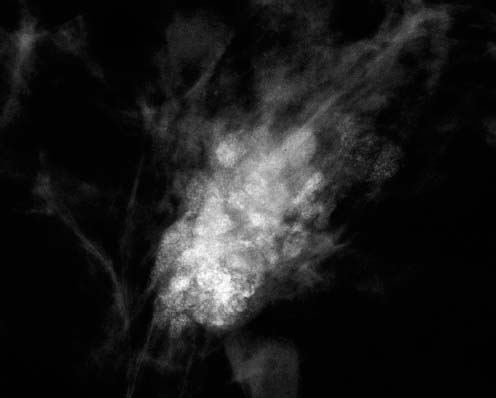
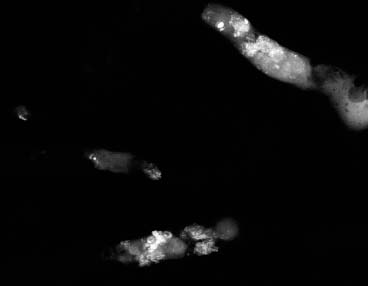
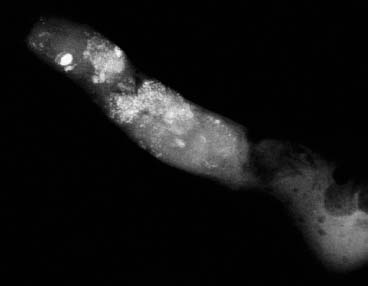
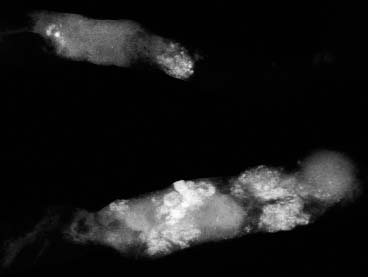
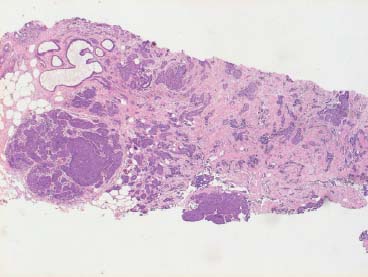
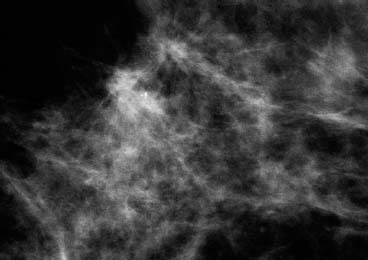
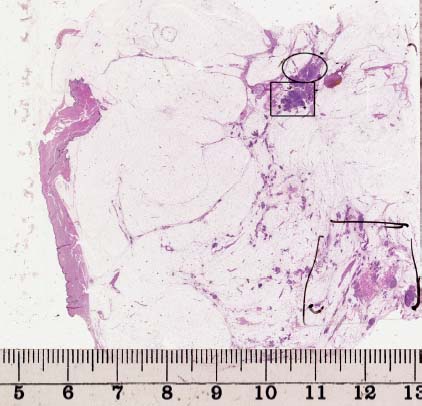
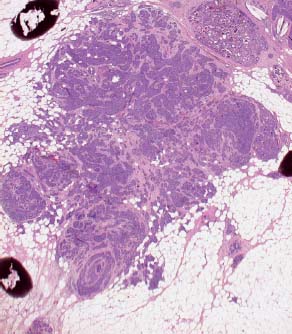
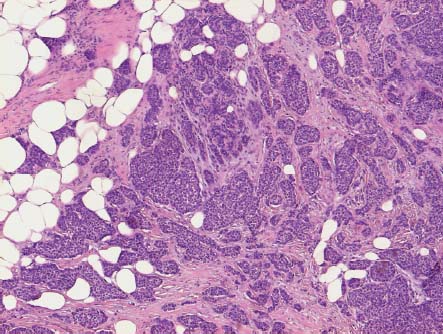
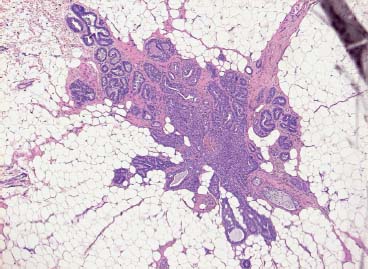
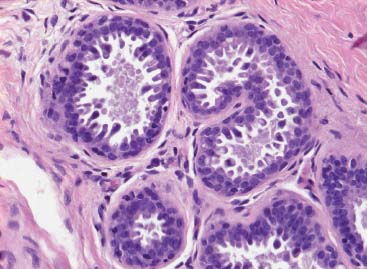
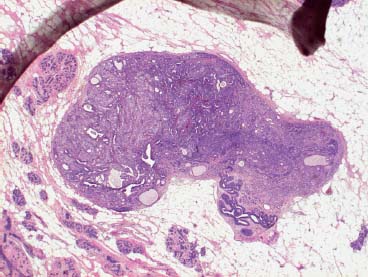
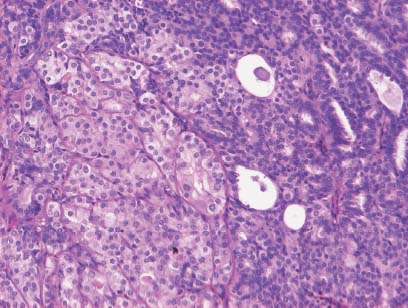
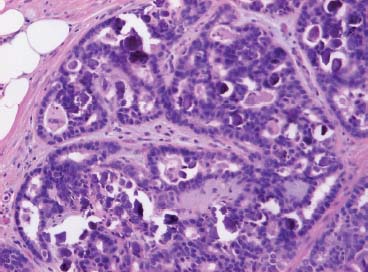
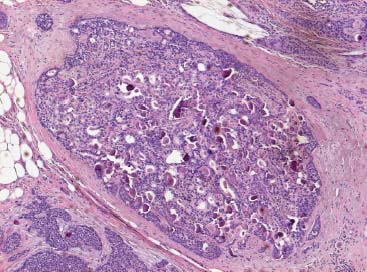
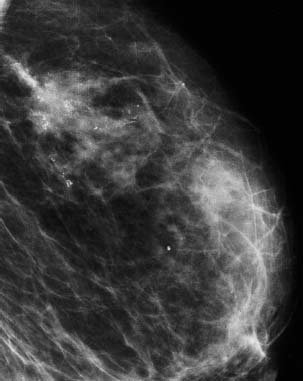
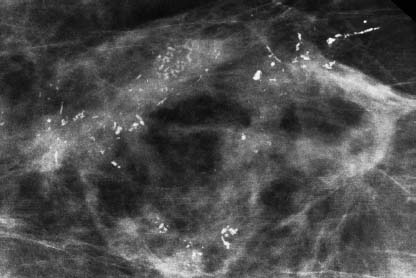
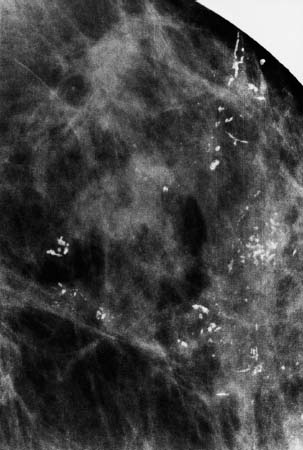
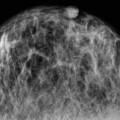
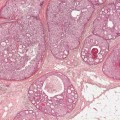
VI Calcifications on the Mammogram Grade 3 ductal carcinoma in situ with casting-type calcifications. Micropapillary carcinoma in situ with skipping stone-like calcifications. Cystically dilated acini with psammoma body-like calcifications. The remaining calcifications are formed within the glandular tissue, that is, within anatomic cavities lined by epithelial cells (terminal ductal lobular units/TDLUs or ducts). Analyzing the distribution of the calcifications on the mammogram will help to determine whether they are located within the TDLU(s) or within the ducts. Linear, fragmented, branching calcifications are located within dilated ducts. The dilatation may be caused by fluid accumulation (plasma cell mastitis/secretory disease-type calcifications) or by proliferation of malignant cells (fragmented or dotted casting-type calcifications). Differential diagnosis of calcifications formed within the ducts is relatively easy. Individual or multiple clusters indicate that the pathological process takes place within the TDLUs, which can be distended by the accumulation of fluid (fibrocystic change) or by malignant cells accompanied by either necrosis (typical of grade 2 in situ carcinoma) or by mucin (typically a product of grade 1 in situ carcinoma cells). Differential diagnosis of calcifications formed within the TDLUs may be difficult when analyzing the mammograms alone. Larger-bore needle biopsy under stereotactic guidance is often essential for differential diagnosis. Once the location of the calcifications has been determined, analysis of the form, size, and density of the individual calcifications will help in distinguishing benign from malignant-type calcifications through a closer understanding of the underlying processes producing them. Microfocus magnification mammography is often essential for this analysis, since it provides higher-resolution images. Microcalcifications are often formed as a byproduct of the epithelial cell proliferation/carcinoma in situ within the TDLUs and/or the ducts. The mammographic appearance of the malignant-type calcifications will be largely dependent upon the malignancy grade of the surrounding cell proliferation and the location of the calcifications (within the TDLUs or ducts). Their distribution will reflect their site of origin—either ducts (linear, scattered within a lobe) or TDLUs (single or multiple clusters). The heterogeneity of in situ carcinoma explains why the malignant-type microcalcifications will be extremely variable in form, size, and density. These are described here in detail. Despite their wide variation in appearance, the malignant-type calcifications can be classified into four basic forms. The corresponding terminology of the American College of Radiology Breast Imaging-Reporting and Data System (BI-RADS) is given in parentheses.1 • Casting-type calcifications. When high-grade carcinoma in situ extensively fills in the ducts and their branches, the central portion of the lumen will contain necrotic cellular debris. Within this necrosis, amorphous calcifications are formed.2 —When the growth pattern of the malignant cells is predominantly solid, the mammographic presentation will be fragmented casting type (BI-RADS: fine linear branching calcifications) (Fig. XXV). These are seen on the mammogram as linear, often branching calcifications with irregular contours. It is the ductal lumen that determines the maximum width of the individual castings. Microfocus magnification views reveal that the calcification fragments differ in density, length, and outline (Cases 90, 92, 96, 99–101, 103–105, 108, 109). The differential diagnosis of the fragmented casting-type calcifications includes only the secretory-type/plasma cell mastitis-type calcifications. Whereas the casting-type calcifications are unilateral and restricted to a single breast lobe, the benign secretory disease-type calcifications are bilateral and widespread. —In cases where the growth pattern of the malignant cells is predominantly micropapillary, dotted casting-type calcifications will be seen on the mammogram. The tips of the ever-growing micropapillary extensions eventually break off and fill up the ductal lumen, where they gradually calcify. The appearance of these calcifications is pathognomonic (Case 102). • Skipping stone-like calcifications in the ducts. When the growth pattern of the malignant cells is micropapillary/cribriform and the cancer cells produce proteinaceous fluid, filling the spherical, intratumoral cavities of the cribriform cancer and distending the ducts containing the micropapillary growths, large, spheroid calcifications may be formed within the fluid. These flat, smooth-contoured calcifications are reminiscent of skipping stones. They fill out a single breast lobe with a fairly uniform intralobar distribution. In this subtype of breast cancer, analysis of the form of the calcifications is of little value and will not lead to the correct diagnosis. Unilateral distribution of skipping stone-like calcifications within a single lobe should raise the suspicion of this special subtype of breast cancer (Case 106). • Crushed stone-like/pleomorphic calcifications (BI-RADS: pleomorphic, heterogeneous).1,3 The individually discernible particles resemble crushed stones or granulated sugar crystals. They are irregular in form, size, and density, and grouped very close together in single (Fig. XXVI) or multiple clusters. The malignant cells and the associated necrosis distend the acini within the TDLU. The amorphous calcifications are formed within this necrosis. Their location within the distended TDLU accounts for their closeness to each other and for their clustered distribution. Since the malignant cells originate within the lobule(s), the term “ductal carcinoma in situ (DCIS)” is a misnomer. These calcifications are typically seen in grade 2 in situ carcinoma, the most frequent form of in situ cancer (Cases 86, 87, 88, 94). Three types of benign, hyperplastic breast changes may present on the mammogram as clustered, discernible, irregular calcifications—fibrocystic change, fibroadenoma, and papilloma. These are the differential diagnostic counterparts for grade 2 in situ carcinoma localized within the TDLUs. Stereotactic percutaneous needle biopsy can provide the correct diagnosis. • Powdery/cotton ball-like calcifications (BI-RADS: amorphous, indistinct).1,3 Psammoma-body-like calcifications may be formed within the mucin secreted by grade 1 in situ carcinoma cells, which proliferate within the acini of the TDLUs. The calcium particles are far too small to be individually perceptible, but the summation of many of them can be seen on the mammogram as multiple clusters of powdery/cotton ball-like calcifications (Cases 95, 97, 98, 107, 120). The BI-RADS terminology for this kind of calcifications is “amorphous,” an unfortunate choice, since the term “amorphous” has long been used by pathologists to describe the calcifications associated with apoptosis within the TDLUs (grade 2 in situ carcinoma) or within the ducts (grade 3 in situ carcinoma). Additionally, these calcifications are not amorphous, since they are crystalline spheres. Use of the same term (amorphous) to describe vastly different calcification types that represent disparate disease processes has the potential of confusing communication between radiologists and pathologists. Multiple clusters of powdery/cotton ball-like calcifications on the mammogram may be seen with either grade 1 in situ carcinoma or sclerosing adenosis. Since both the benign, hyperplastic breast change (sclerosing adenosis) and the malignant process (grade 1 in situ carcinoma) develop within the TDLUs and both are associated with similarly appearing psammoma body-like calcifications, radiologic differentiation of these two entities is not possible. Multiple cluster powdery calcifications seen on the mammogram will have an approximately 50% probability of representing grade 1 in situ cancer (Cases 97, 98, 107). These calcifications arise within the lumens of the TDLU(s) or duct(s), and are thus limited in size to these dimensions—hence, the term microcalcifications. Density analysis should include a comparison of the densities of the individual particles with each other (interparticulate density analysis). The crushed stone-like/pleomorphic and casting-type calcifications both show great variations in density among adjacent particles. Although the actual number of calcifications has been considered by some to have diagnostic significance, the distribution, form, size, and density of the calcifications are of far greater importance. Magnification mammography in particular has demonstrated that the number of calcifications visible can be highly dependent upon the mammography technique. The number of crushed stone-like/pleomorphic calcifications may vary considerably from cluster to cluster, the number of calcification particles in the dotted casting type are innumerable, and the powdery/cotton ball-like calcifications are not countable. It is important to note that the casting-type calcifications are so characteristic of grade 3 in situ carcinoma, that the diagnosis can be made on the basis of one or two such calcifications alone (Cases 101, 105). Fig. XXV Diagram of an extremely distended duct with solid-cell proliferation, central necrosis, and amorphous, casting-type calcifications. Fig. XXVI Crushed stone-like calcifications within a TDLU. The individual particles are irregular in size, shape, and density, and are grouped in a cluster. A 48-year-old asymptomatic woman. Two consecutive screening examinations. Mammography First screening examination. Fig. 86 a: Right breast, detailed view of the mediolateral oblique (MLO) projection. Normal mammogram. Second screening examination, 24 months later. No palpable tumor. Fig. 86 b: Right breast, detailed view of the MLO projection. A cluster of microcalcifications is now seen in the upper half of the breast (arrow). No associated tumor mass is demonstrable. Fig. 86 c, d: Right breast, microfocus magnification views, MLO and craniocaudal (CC) projections. Analysis of the Calcifications The de novo, tiny, crushed stone-like/pleomorphic calcifications are irregular in form, size, and density and occur in a compact cluster. This is a typical mammographic image of malignant-type, crushed stone-like/pleomorphic calcifications within a single TDLU. Fig. 86 e, f: Operative specimen radiographs, magnification view. Histology In situ carcinoma with necrosis within a TDLU. Histology also reveals invasion. Follow-up The woman was still alive 19 years later. Asymptomatic 50-year-old woman. First screening study. Physical Examination No palpable tumor in the breasts. Mammography Fig. 87 a: Left breast, MLO projection. Two clusters of microcalcifications are seen in the upper half of the breast (arrow). In addition, a solitary, 4-mm eggshell-like calcification is seen in the central portion of the breast, mammographically benign. Fig. 87 b, c: Magnification view, MLO projection and specimen radiography. Analysis of the Clustered Calcifications Distribution: cluster; the calcifications are seen very near to each other in a small area of the breast Form: crushed stone-like/pleomorphic, some elongate; highly irregular Density: variable Conclusion Mammographically malignanttype, crushed stone-like/pleomorphic microcalcifications. In situ carcinoma within TDLUs; solid-cell proliferation, intraluminal necrosis and amorphous calcifications within the distended acini. Fig. 87 d: Low-power view of the grade 2 carcinoma in situ involving several TDLUs (hematoxylin and eosin [H&E], 40 ×). Fig. 87 e: The acini from one TDLU filled with malignant cells, corresponding to one cluster on the mammogram (H&E, 100 ×). Fig. 87 f: Higher-power magnification of a single acinus showing the cellular details of a grade 2 carcinoma in situ (H&E, 200 ×). Follow-up The woman died 15 years later from myocardial infarction, with no evidence of breast cancer at the time of death. This 68-year-old woman felt a thickening in the lateral portion of her left breast. Mammography Fig. 88 a, b: Left breast, CC projection, detailed view of the contact mammogram and microfocus magnification view. Innumerable calcifications of varying form, size, and density. They are a mixture of the casting-type (long, fragmented, some of them branching) and the skipping stone-like (fairly large, high-density, some oval-shaped) calcifications, and are surrounded by an ill-defined density. Conclusion Mammographically malignant-type calcifications with an associated ill-defined, non-specific density. Histology Infiltrating ductal carcinoma, with an extensive associated in situ component. Fig. 88 c: Low-power view of the micropapillary ductal carcinoma in situ component (H&E, 40 ×). Fig. 88 d, e: Higher magnification of the grade 2 micropapillary carcinoma in situ. The ducts are distended by the fluid produced by the cancer cells. Within this fluid, skipping stone-like calcifications are seen randomly, corresponding to the larger calcifications on the mammogram (H&E, 200 × and 300 ×). Fig. 88 f: Cellular details of this grade 2 micropapillary ductal carcinoma in situ with a portion of the fluid-filled lumen (H&E, 400 ×). Follow-up The woman was still alive 19 years later at the age of 87 years, with no evidence of breast cancer. Mammography Fig. 89 a: Right breast, detailed view of the CC projection. Fig. 89 b: Microfocus magnification view. There are numerous fragmented casting-type calcifications, indicating the presence of malignancy in a large volume of the breast. Histology Grade 3 in situ carcinoma with solid-cell proliferation, central necrosis and amorphous calcifications. No histologic signs of invasion. Comment The disorganized, haphazard arrangement of the calcifications that outline the duct-like structures indicates the presence of neoductgenesis.2 This 27-year old woman felt a lump in her left breast. Mammography Fig. 90 a: Detailed view of the MLO projection, left breast. Fig. 90 b: Enlarged view of the portion of the left breast containing the palpable tumor. There are numerous fragmented casting-type, branching calcifications associated with architectural distortion. The findings are characteristic of a malignant breast tumor. Histology Infiltrating carcinoma with lymph node metastases. Fig. 90 c: Low-power view of the invasive ductal carcinoma including an in situ component (H&E, 40 ×). Fig. 90 d: Poorly differentiated invasive ductal carcinoma (H&E, 200 ×). Fig. 90 e: Axillary lymph node containing metastases (H&E, 200 ×). Follow-up The patient died 2 years later of metastatic breast carcinoma, aged 29 years. Comment The patient had a classic, poorly differentiated invasive ductal carcinoma in addition to invasive, poorly differentiated duct-forming invasive cancer, revealed by the casting-type calcifications. This large tumor burden was fatal. Routine screening examination of this 62-year-old asymptomatic woman. Mammography Fig. 91 a, b: Right breast, detailed view of the CC projection (a) and microfocus magnification view (b). There are innumerable branching, fragmented casting-type calcifications of varying length and density. An ill-defined density surrounds the calcifications. This may correspond to either infiltration or desmoplastic reaction. Conclusion The presence of fragmented casting-type calcifications associated with a large ill-defined density is pathognomonic for an aggressive, highly malignant cancer. Histology Invasive ductal carcinoma associated with high-grade ductal carcinoma in situ with central necrosis. Fig. 91 c: Low-power histologic image demonstrates a large number of tightly packed, cancer-filled duct-like structures with central necrosis and amorphous calcifications corresponding to the casting-type calcifications on the mammogram (H&E, 40 ×). Comment Both the mammographic and histologic image are characteristic for neoductgenesis.2 Follow-up The woman died 13 years later from myocardial infarction, aged 74 years. There was no evidence of breast cancer. Mammography Fig. 92 a–d: Microfocus magnification mammography of four cases of fragmented, casting-type, mammographically malignant-type calcifications. The calcifications differ in density, width, and length, and are irregular in outline. They are tightly packed and point in all directions, forming a disorganized pattern characteristic of neoductgenesis. An asymptomatic woman aged 40 years. First screening examination. Physical Examination No palpable tumor in the breasts. Mammography Fig. 93 a, b: Left breast, detailed views of the MLO and CC projections. There is a small group of calcifications in the lower outer quadrant, without an associated tumor mass. Fig. 93 c: Microfocus magnification view of the are a with calcifications in the MLO projection. Analysis This is an additional example of fragmented and dotted casting-type calcifications. These are formed within segments of a duct and its branches. The ductal lumen contains the grade 3 in situ carcinoma cells, central necrosis, and amorphous calcification fragments of varying length, density, and outline. Fig. 93 d: Operative specimen radiograph, magnification. Histology Invasive and in situ ductal carcinoma. No lymph node metastases. Fig. 93 e: Overview of the 3-mm invasive component associated with an in situ focus (H&E, 40 ×). Fig. 93 f: Higher-power histologic image of the invasive tumor (H&E, 220 ×). Fig. 93 g, h: Cellular details of the extensive grade 3 ductal carcinoma in situ (H&E, 600 ×). Fig. 93 i: Overview of the retromamillary area with extension of the high-grade ductal carcinoma in situ (H&E, 12.5 ×). Fig. 93 j: High-power view of the retromamillary ductal carcinoma in situ (H&E, 600 ×). Follow-up The woman was still alive 20 years later at the age of 60 years. An asymptomatic 75-year-old woman. First screening study. Physical Examination No palpable tumor in the breasts. Mammography Fig. 94 a, b: Left breast, detailed views of the MLO and CC projections. In the upper outer quadrant there are two clusters of calcifications surrounded by an ill-defined density. Fig. 94 c, d: Microfocus magnification views, MLO and CC projections. Analysis of the Calcifications Distribution: cluster Form: crushed stone-like/pleomorphic, variable in shape Density: highly variable, some fade into the background Conclusion Mammographically malignant-type calcifications within an ill-defined density. The cluster distribution suggests that the malignant process is confined to the TDLU. Histology In situ carcinoma with minimal invasion. Fig. 94 e: Low-power magnification of this grade 2 carcinoma in situ within a TDLU. The amorphous calcifications correspond to the microcalcifications seen on the mammogram (H&E, 20 ×). Fig. 94 f: Higher-magnification histology image showing a single acinus with solid proliferation of malignant cells (H&E, 200 ×). Follow-up The woman died 12 years later from pneumonia at the age of 87 years, with no evidence of breast cancer at the time of death. This 45-year-old asymptomatic woman was called back from mammography screening for assessment of the multiple cluster calcifications detected in the upper outer quadrant of her left breast. Mammography and Ultrasound Fig. 95 a, b: Left breast, detail of the MLO projection (a) and microfocus magnification (b) of the region containing the multiple clusters of powdery calcifications. Fig. 95 c, d: Left breast, detail of the CC projection (c) and microfocus magnification view (d) of the region in the rectangle. The numerous powdery/cotton ball-like calcifications are hidden in extremely dense fibrosis. No tumor mass is demonstrable. Fig. 95 e: Left breast, additional detail of the CC projection, microfocus magnification view. Fig. 95 f: Hand-held ultrasound helps to detect mammographically occult small, simple cysts. Fig. 95 g: Specimen radiograph following preoperative localization using the bracketing technique. Fig. 95 h–k: Specimen slice radiographs. There are innumerable clusters of powdery/cotton ball-like calcifications throughout each slice. In addition, there are a few clusters containing discernible, round, high-density calcifications. No tumor mass is demonstrable. Sclerosing adenosis without malignancy. Fig. 95 l: Large-section histology, low-power image. The abnormal tissue (between the dashed lines) occupies an area of 5 cm. Fig. 95 l–q: Low and intermediate power histologic images (H&E). The psammoma-body-like calcifications are localized within lobules, associated with sclerosing adenosis. There is no epithelial cell atypia, and no malignancy was found. An asymptomatic woman, aged 61 years. First screening study. Physical Examination No palpable tumor in the breasts. Mammography Fig. 96 a: Right breast, MLO projection. A cluster of calcifications is seen in the axillary portion of the breast (arrow), with no associated tumor mass. Fig. 96 b: Right breast, microfocus magnification view, MLO projection. Analysis Distribution: cluster Form: a mixture of crushed stone-like/pleomorphic and fragmented casting-type calcifications Density: varies between high density and barely visible Size: microcalcifications of variable size. A few elongated, casting-type calcifications are evident Conclusion Mammographically malignant-type calcifications. The casting-type calcifications are pathognomonic for a high-grade in situ carcinoma. Histology Carcinoma in situ without a demonstrable invasive component. Fig. 96 c: Grade 3 carcinoma in situ with central necrosis and amorphous calcifications, with surrounding desmoplastic reaction and lymphocytic infiltration (H&E, 20 ×). Fig. 96 d: Higher-power magnification of a single, cancerous duct (H&E, 300 ×). Follow-up The woman was still alive 20 years later, with no evidence of breast cancer. This 48-year-old asymptomatic woman was called back for further assessment of a small group of powdery calcifications detected in the lower portion of the right breast. Mammography Fig. 97 a, b: Right breast, detail of the MLO projection (a) and microfocus magnification of the area with the powdery calcifications (b). Fig. 97 c: An additional microfocus magnification image of the region with the powdery calcifications. Fig. 97 d: Specimen radiograph of the large-bore percutaneous needle biopsy. A large number of calcifications are included in the biopsy specimen. Fig. 97 e–g: Histology of the percutaneous large-bore needle biopsy: one dilated TDLU contains low-grade cancer in situ and psammoma body-like calcifications corresponding to the microcalcifications seen on the mammogram. Fig. 97 h, i: Histology of the surgical specimen: one TDLU is distended by low-grade carcinoma in situ without associated calcifications (occult on mammography). multifocal grade 1 carcinoma in situ. Analysis of the Calcifications Distribution: cluster Form: powdery, cotton ball-like Density: very faint Conclusion Microscopic diagnosis in each powdery calcification case is necessary because sclerosing adenosis and grade 1 in situ carcinoma cannot be differentiated solely by radiologic imaging, since the calcifications associated with both of these diseases are identical. This 78-year-old woman was operated in her left breast at the age of 70 years, for a 9 × 6 mm invasive lobular cancer associated with grade 1 in situ carcinoma and lobular carcinoma in situ over an area measuring 50 × 60 mm. Eight years later, her right breast mammograms showed a de novo, oval, lobulated density associated with powdery calcifications. Physical Examination No palpable tumor in the breasts. Mammography Fig. 98 a, b: Right LMO (a) and CC (b) projections. The de novo density associated with powdery calcifications is seen encircled. Fig. 98 c, d: Microfocus magnification views on the LM (lateromedial) (c) and (d) CC projections. An ill-defined, unusually lobulated tumor mass contains numerous clusters of powdery calcifications. Conclusion An ill-defined, lobulated tumor associated with powdery calcifications is, with high probability, a malignant breast tumor associated with low-grade in situ carcinoma. Fig. 98 e–g: Radiographs of the vacuum-assisted biopsy specimen. Fig. 98 h: Histologic examination of the percutaneous biopsy specimen reveals invasive ductal carcinoma and grade 1 in situ cancer. Fig. 98 i, j: Operative specimen radiographs. Histology 9 × 6 mm moderately differentiated invasive ductal carcinoma associated with grade 1 in situ cancer (Fig. 98h) and lobular cancer in situ on an area measuring 60 × 50 mm. None of the three surgically removed sentinel nodes showed signs of metastases. Fig. 98 l, m: Low- and intermediate-power histology images of the moderately differentiated invasive carcinoma component (within the area marked with a rectangle on Fig. 98 k). Fig. 98 n, o: Low- and high-power histology images of the associated in situ carcinoma (within the area marked with an oval on Fig. 98 k). Fig. 98 p, q: Low- and intermediate-power images of in situ carcinoma foci 45 mm from the invasive carcinoma. Fig. 98 r, s: Psammoma body-like calcifications within the in situ cancer localized close to the invasive cancer. These correspond to the powdery calcifications on the mammogram. A 74-year-old woman, not aware of any breast abnormality. First screening examination. Mammography Fig. 99 a: Left breast, detailed view of the MLO projection. In the upper outer quadrant there is a 5 × 5 cm area containing numerous calcifications associated with a non-specific, ill-defined density. Fig. 99 b, c: Microfocus magnification views, MLO and CC projections. Most of the calcifications are of the fragmented casting type, mammographically malignant. Analysis This case gives an excellent opportunity to demonstrate the fragmented casting-type calcifications. Their shape is determined by the uneven production of calcification within the irregular central necrosis. The lumen contains consecutive fragments; the contour of these calcifications is irregular, and their density, although high, varies from particle to particle. A calcification may be branching when it extends into adjacent branches of a duct. Histology Poorly differentiated invasive ductal carcinoma associated with a grade 3 ductal carcinoma in situ. No lymph node metastases were detected at the time of surgery. Note The infiltration probably accounts for some of the density surrounding the calcifications. Follow-up The patient died 2 years 5 months later of metastatic breast carcinoma, aged 76 years. Comment This is a typical example of neoductgenesis where the newly formed duct-like structures are filled with high-grade carcinoma cells, necrosis, and casting-type calcifications. These consist of poorly differentiated “duct-forming invasive carcinoma” (rather than in situ cancer), and together with the associated conventional invasive carcinoma, the patient was subjected to a large, aggressive tumor burden.2 A 73-year-old asymptomatic woman. First screening examination. Mammography Fig. 100 a: Left breast, CC projection. A group of calcifications is seen in the central portion of the breast (arrows). Fig. 100 b: Microfocus magnification view of the region with microcalcifications, CC projection. Analysis Typical intraductal, casting-type calcifications. They are irregular in shape, size, and density, and follow the course of a duct and its branches. Conclusion Mammographically malignant-type calcifications. Histology
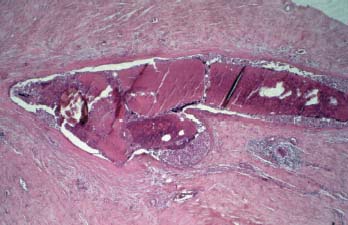
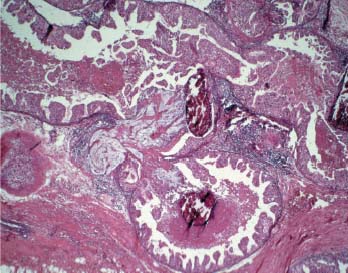
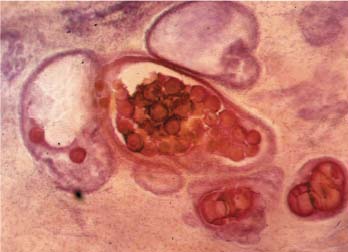
Malignant-Type Calcifications within Ducts and/or in Terminal Ductal Lobular Units
Form
Size
Density
Number
Calcifications Localized within Ducts
Calcifications Localized within Terminal Ductal Lobular Units
Practice in Calcification Analysis
86
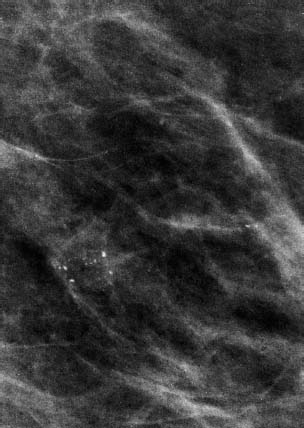
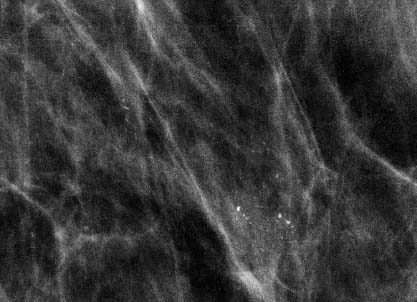
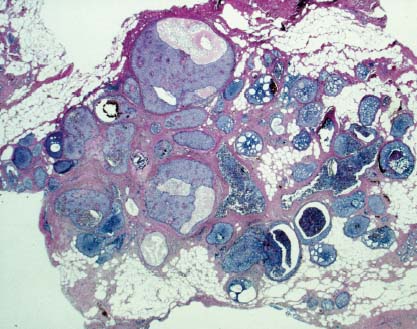
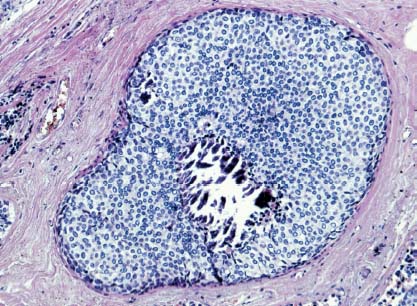
87
88
89
90
91
92
93
94
95
96
97
98
99
100
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree