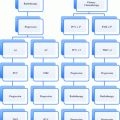
Primary tumor (T)
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Tis
High-grade dysplasia (HGD)
T1
Tumor invades lamina propria, muscularis mucosae, or submucosa
T1a
Tumor invades lamina propria or muscularis mucosae
T1b
Tumor invades submucosa
T2
Tumor invades muscularis propria
T3
Tumor invades adventitia
T4
Tumor invades adjacent structures
T4a
Resectable tumor invading pleura, pericardium, or diaphragm
T4b
Unresectable tumor invading other adjacent structures, such as aorta, vertebral body, trachea, etc.
Regional lymph nodes (N)
NX
Regional lymph node(s) cannot be assessed
N0
No regional lymph node metastasis
N1
Metastasis in 1–2 regional lymph nodes
N2
Metastasis in 3–6 regional lymph nodes
N3
Metastasis in seven or more regional lymph nodes
Distant metastasis (M)
M0
No distant metastasis
M1
Distant metastasis
Histologic grade (G)
GX
Grade cannot be assessed—stage grouping as G1
G1
Well differentiated
G2
Moderately differentiated
G3
Poorly differentiated
G4
Undifferentiated—stage grouping as G3 squamous
Table 2
Prognostic groups by TNM stage/anatomic stage for squamous cell carcinoma UICC-AJCC 7th edition (Rice et al. 2010)
Anatomic stage/prognostic groups | |||||
|---|---|---|---|---|---|
Squamous cell carcinoma | |||||
Stage | T | N | M | Grade | Tumor location |
0 | Tis (HGD) | N0 | M0 | 1, X | Any |
IA | T1 | N0 | M0 | 1, X | Any |
IB | T1 | N0 | M0 | 2–3 | Any |
T2–3 | N0 | M0 | 1, X | Lower, X | |
IIA | T2–3 | N0 | M0 | 1, X | Upper, middle |
T2–3 | N0 | M0 | 2–3 | Lower, X | |
IIB | T2–3 | N0 | M0 | 2–3 | Upper, middle |
T1–2 | N1 | M0 | Any | Any | |
IIIA | T1–2 | N2 | M0 | Any | Any |
T3 | N1 | M0 | Any | Any | |
T4a | N0 | M0 | Any | Any | |
IIIB | T3 | N2 | M0 | Any | Any |
IIIC | T4a | N1–2 | M0 | Any | Any |
T4b | Any | M0 | Any | Any | |
Any | N3 | M0 | Any | Any | |
IV | Any | Any | M1 | Any | Any |
Table 3
TNM staging of esophageal and esophagogastric junction (EGJ) adenocarcinoma UICC-AJCC 7th edition (Rice et al. 2010)
Primary tumor (T) | |
TX | Primary tumor cannot be assessed |
T0 | No evidence of primary tumor |
Tis | High-grade dysplasia (HGD) |
T1 | Tumor invades lamina propria, muscularis mucosae, or submucosa |
T1a | Tumor invades lamina propria or muscularis mucosae |
T1b | Tumor invades submucosa |
T2 | Tumor invades muscularis propria |
T3 | Tumor invades adventitia |
T4 | Tumor invades adjacent structures |
T4a | Resectable tumor invading pleura, pericardium, or diaphragm |
T4b | Unresectable tumor invading other adjacent structures, such as aorta, vertebral body, trachea, etc. |
Regional lymph nodes (N) | |
NX | Regional lymph node(s) cannot be assessed |
N0 | No regional lymph node metastasis |
N1 | Metastasis in 1–2 regional lymph nodes |
N2 | Metastasis in 3–6 regional lymph nodes |
N3 | Metastasis in seven or more regional lymph nodes |
Distant metastasis (M) | |
M0 | No distant metastasis |
M1 | Distant metastasis |
Histologic grade (G) | |
GX | Grade cannot be assessed—stage grouping as G1 |
G1 | Well differentiated |
G2 | Moderately differentiated |
G3 | Poorly differentiated |
G4 | Undifferentiated—stage grouping as G3 squamous |
Table 4
Prognostic groups by TNM stage/anatomic stage for adenocarcinoma UICC-AJCC 7th edition (Rice et al. 2010)
Anatomic stage/prognostic groups | ||||
|---|---|---|---|---|
Adenocarcinoma carcinoma | ||||
Stage | T | N | M | Grade |
0 | Tis (HGD) | N0 | M0 | 1, X |
IA | T1 | N0 | M0 | 1–2, X |
IB | T1 | N0 | M0 | 3 |
T2 | N0 | M0 | 1–2, X | |
IIA | T2 | N0 | M0 | 3 |
IIB | T3 | N0 | M0 | Any |
T1–2 | N1 | M0 | Any | |
IIIA | T1–2 | N2 | M0 | Any |
T3 | N1 | M0 | Any | |
T4a | N0 | M0 | Any | |
IIIB | T3 | N2 | M0 | Any |
IIIC | T4a | N1–2 | M0 | Any |
T4b | Any | M0 | Any | |
Any | N3 | M0 | Any | |
IV | Any | Any | M1 | Any |
These tumors, at the esophagogastric junction (EGJ) and proximal 5 cm of the stomach that extend into the EGJ or esophagus, are staged as esophageal cancers (Table 5). While all other tumors with an epicenter in the stomach >5 cm from the EGJ, or those within 5 cm of the EGJ without extension into the esophagus are staged as gastric cancers. A sub classification of these junctional tumors can be made by the classification described by Siewert and Stein (1998) (Table 6).
Table 5
Primary site of esophageal cancer based on proximal edge of tumor according to the UICC-AJCC 7th edition (Rice et al. 2010)
Anatomic name | Esophageal location | Anatomic boundaries | Endoscopic distance from incisors |
|---|---|---|---|
Cervical | Upper | Hypopharynx to sternal notch | 15 to <20 cm |
Thoracic | Upper | Sternal notch to azygos vein | 20 to <25 cm |
Middle | Lower border of azygos vein to inferior pulmonary vein | 25 to <30 cm | |
Lower | Lower border of inferior pulmonary vein to esophagogastric junction | 30 to <40 cm | |
Abdominal | Lower | Esophagogastric junction to 5 cm below esophagogastric junction | 40–45 cm |
Esophagogastric junction/cardia | Esophagogastric junction to 5 cm below esophagogastric junction | 40–45 cm |
Type | Description |
|---|---|
Type I | Located between 5 and 1 cm proximal to the anatomical cardia. Adenocarcinoma of the distal esophagus that usually arises from an area with specialized intestinal metaplasia |
Type II | Located between 1 cm proximal and 2 cm distal to the anatomical cardia. True carcinoma of the cardia arising from the cardiac epithelium or short segments with intestinal metaplasia |
Type III | Located between 2 and 5 cm distal to the anatomical cardia. Subcardial gastric carcinoma that infiltrates the EGJ and distal esophagus from below |
A validation study of this 7th edition of the UICC-AJCC staging system was performed by Talsma et al., which showed for surgical esophageal cancer patients that the 7th edition of staging provided more accurate prognostic stratification for OS in comparison to the 6th edition (Talsma et al. 2012).
2.3 Imaging
Imaging modalities as EUS, CT, PET-CT and MRI currently play an important role in staging and in patient selection before CRT. They may also be utilized for post CRT clinical re-staging (ycTNM) after induction treatment or response monitoring during treatment, however the results should be interpreted with caution in this setting (Ribeiro et al. 2006). During these different phases of the patient care some have attempted to identify a distinctive role for each modality.
2.3.1 EUS
2.3.1.1 Staging
Endoscopic ultrasound (EUS) uses a high frequency ultrasound transducer to obtain detailed images of the tumor mass and the relationship with the five-layered structure of the esophageal wall. EUS attempts to provide measurements of tumor thickness and is regularly used to estimate tumor extension in initial staging for esophageal cancer (Ribeiro et al. 2006). The discriminatory power for distinguishing between early stage tumors and those with deeper invasion may approach 80–90 %. Some have found this gross distinction prognostic in identifying those patients at risk for a positive circumferential resection margin (CRM), if treated with isolated surgery (Reid et al. 2012). However, its exact TNM accuracy is the least prognostic of available clinical information in predicting pre operative stage (Reid et al. 2012; van Vliet et al. 2008; Thosani et al. 2012).
While, EUS remains a frequently used clinical estimate of primary tumor staging, there are technical limitations to its ubiquitous use. Not all patients are capable of receiving a complete EUS due to esophageal stenosis. Additionally, the accuracy of EUS is operator dependent and is subject to a learning curve (Fockens et al. 1996). Ultimately, preoperative staging of lymph node status is challenging. A recently proposed tool from Gaur et al. (2010) for predicting pathologic lymph node involvement based on clinical information is discussed in further detail below in Nomograms and Predictive models section.
2.3.1.2 Re-staging
For response evaluation, EUS continues to be used as the primary diagnostic modality. However, the accuracy of EUS restaging varies significantly across several recent retrospective analyses (Ribeiro et al. 2006; Giovannini et al. 1997; Chak et al. 2000).
Different methods have been proposed for response assessment with EUS. The first method is to restage according to the TNM staging system (Fockens et al. 1996), second method is to measure the relative reduction in thickness of the tumor (Gaur et al. 2010; Giovannini et al. 1997) and a third method is to measure the relative tumor shrinkage at the maximum cross-sectional area (MCSA) (Gaur et al. 2010; Chak et al. 2000). However, with these different methods the accuracy is still poor and ranges from 17 to 59 % (Sloof 2006; Hirata et al. 1997; Zuccaro et al. 1999; Bowrey et al. 1999). Even when a EUS is combined with biopsy, the accuracy does not exceed 31 % to correctly predict a pCR (Sarkaria et al. 2009). Of patients that have a negative biopsy on restaging endoscopy (cCR) less than 30 % will have a pCR (Sarkaria et al. 2009).
A possible explanation for this discrepancy between endoscopic staging and subsequent pathologic staging is that EUS may not be able to differentiate between post-treatment inflammation or fibrosis and residual tumor (Jamil et al. 2008).
2.3.2 CT
2.3.2.1 Staging
Computed tomography (CT) is usually one of the first steps in staging esophageal cancer patients and is used to evaluate the region of the primary tumor and evaluate for distant metastases. However, the accuracy for locoregional staging is limited. Accuracy for tumor staging has been reported with a range of 42–68 % (Lowe et al. 2005; Wu et al. 2003) and for regional lymph node metastases the pooled sensitivity and specificity is only 0.50 (95 % C.I. 0.41–0.6) (van Vliet et al. 2008). Also, when used for screening for distant metastases CT-scans have difficulty recognizing small distant metastases, while a PET-CT is more sensitive (van Vliet et al. 2008).
2.3.2.2 Re-staging
CT scan is the most commonly used diagnostic modality in monitoring response of nonsurgical therapy for solid tumors. For esophageal cancer restaging, however, its role remains ambiguous. CT gives good visualization of the tumor bulk in majority of the patients. However, when tumor shrinkage is correlated to pathological response following neoadjuvant treatment some have found a clear correlation (Swisher et al. 2004; Voncken et al. 2012), while others failed (Griffith et al. 1999; Jones et al. 1999).
This difference could be due to an overestimation of edema, inflammation and fibrosis for residual tumor (Westerterp et al. 2005). While, CT remains one of many modalities in re-staging after induction treatment, primary tumor response should be interpreted with caution.
2.3.3 PET
FDG-PET is a nuclear imaging modality that evaluates tumor physiology and allows for a quantitative functional assessment of the primary via the standardized uptake value (SUV). Nearly all primary esophageal cancers have high levels of cellular metabolism, increased glycolysis, and an increased number of glucose transporters. In almost all cases, SCC primary tumors have a high uptake of FDG. In adenocarcinomas this FDG accumulation is more variable, with a minority (6 %) of the tumors being non-avid, usually the mucous containing and poorly differentiated tumor types or tumors too small to detect (<5 mm) by FDG-PET (Wagner et al. 2009; Stahl et al. 2003; Wong and Chambers 2008).
2.3.3.1 Staging
FDG-PET can provide information in initial staging, especially for finding regional nodal metastases and silent distant metastases, where it has a role in selecting patients that will benefit from neoadjuvant CRT. In addition, FDG-PET can contribute to localization, size measurement and GTV definition of the primary tumor and lymph nodes (Katsoulis et al. 2007).
2.3.3.2 Restaging
PET-CT provides several pieces of clinically relevant information in restaging after induction treatment. A PET-CT can detect occult metastases after induction treatment and thus saving patients from undergoing a non-curative esophagectomy. A PET-CT after induction treatment detects metastases in 8 % of patients with a consequent adjustment in therapeutic plan (Bruzzi et al. 2007).
An additional advantage of response monitoring with PET-CT is the prognostic value in the decrease, or lack there of, in SUV of the primary tumor. While a PET-directed therapy does have the potential to change clinical practice and improve outcomes, it cannot currently be considered a standard approach in isolation. Standardization of quantitative results across facilities continues to be a technical roadblock. Several methods have been proposed as standard procedure for metabolic response analysis using the standard uptake value (SUV) as a semi quantitative measure of FDG uptake. Proposed methods are: SUV pretreatment, SUV after chemoradiation, percentage of decline of SUV, attainment of a metabolic complete response after chemoradiation and to show an early metabolic response 14 days after start of chemoradiation.
These different methods were analyzed in a systematic review of Omloo et al. evaluating 31 studies (Omloo et al. 2011). Fifteen of these studies tested the pretreatment FDG uptake as a predictive factor. On univariate analysis, SUV was a predictor of survival in 12 out of 15 studies and multivariate analysis showed only in two out of eight studies that SUV was an independent predictor of survival. SUV decrease after completion of neoadjuvant treatment was predictive in only two out of six studies. Finally, there were six studies looking at the SUV decrease and prognosis early during neoadjuvant therapy. SUV decrease was a predictor of response in all of these six studies and a predictor of survival in five of these six studies.
Comparative analysis across FDG-PET articles is challenging due the non-standardization of the image acquisition process and subsequent analytic thresholds. Since the methodology for image acquisition varies, the SUV threshold to predict prognosis varies significantly between analyses (from 3 to 10.5) and cutoff values for amount of SUV change differentiating responders from non-responders also varies depending on the publication (from −30 to −70 %) (Omloo et al. 2011). This illustrates the difficulty to translate these results to clinical practice, although the field of treatment stands to benefit from an adequately powered prospective trial evaluating the true relevance of early SUV decline during CRT. In conclusion, early SUV response assessment holds promise to potentially guide ongoing treatment, but the implementation and technical applicability have not yet developed to the extent required to find a clinical role for routine use.
2.3.4 MRI
The recent development of functional MRI imaging has opened a new window of opportunities for staging esophageal tumors, monitoring response to treatment and potentially even predicting biological behavior (Chang 2009; Riddell et al. 2007). Esophageal imaging with MRI has some technological challenges due to local cardiorespiratory motion artifact. However, with an accurately tuned sequence accurate images can be acquired.
For staging the esophageal tumor, EUS is the modality of first choice, however for 6 % of newly diagnosed patients, EUS is not possible due to a narrowing of the esophageal lumen and subsequent inability to pass the endoscope. CT is less accurate in differentiating depth of tumor invasion, thus for staging those patients, MRI could be an alternative (Riddell et al. 2007).
Staging the depth of tumor growth with MRI has an accuracy of about 60 % (Jamil et al. 2008), but with the MRI technique still under development, imaging reaches a higher level of precision, however this has not yet been correlated with accuracy of overall stage (Riddell et al. 2007). MRI cannot differentiate each layer of the esophageal wall, therefore an alternative T differentiation standard is described by Botet et al. (1991) and by Riddell et al. (2007) (Table 7).
Staging according to Botet | MRI features defined by Riddell | |
|---|---|---|
T stage | ||
T1 | Thickening less than 5 mm | No discernable tumor |
T2 | Thickening of the wall greater than 5 mm and less than 15 mm | Intermediate signal intensity within the high signal submucosa and muscularis propria (low signal). Low signal outer margin of muscularis propria clearly defined and remains intact |
T3 | Thickening of the wall greater than 15 mm with irregularity of the outer margin | Nodular irregularity of the outer margin of the muscularis propria. Intermediate signal intensity nodules extending from the esophageal wall into the peri-esophageal tissues |
T4 | Tumor invasion of adjacent structures such as the trachea, aortic pericardium, or vertebral body | Intermediate signal intensity tumor extending into adjacent structures. Loss of a high signal fat plane between intermediate signal intensity tumor and an adjacent structure |
N stage | ||
N0 | Lymph nodes less than 10 mm in diameter were considered benign nodes | Uniform high signal intensity returned from peri-esophageal tissues |
N1 | Lymph nodes greater than 10 mm in short axis diameter were considered abnormal | Nodular intermediate signal intensity nodules >2 mm in size within the peri-esophageal tissues |
Recently developed MRI techniques such as diffusion weighted imaging (DWI) and dynamic contrast-enhanced (DCE) MRI may provide a relative increased accuracy in clinical staging and response assessment of esophageal tumors.
In diffusion-weighted magnetic resonance imaging (DWI) each voxel reflects the amount of water diffusion at that location. This diffusion process can be quantified by measuring the apparent diffusion coefficients (ADCs) of a voxel. ADC measurements have been suggested for staging or as predictive markers. However, its role for staging looks not as promising as its role as a predictor (Sakurada et al. 2009; Aoyagi et al. 2011). Further investigation is warranted to determine the exact role of DWI.
Dynamic contrast enhanced (DCE-) MRI has the ability to show alterations of vascular integrity that result from pathologic angiogenesis. Esophageal cancer is associated with a higher vascularization and an increase in vascular density, compared with normal esophageal tissue. In DCE-MRI, after a bolus of gadolinium chelate is administered intravenously, flow signal and leak can be observed. Two parameters are of importance, the contrast reagent transfer between plasma and interstitial space (Ktrans) and the volume fraction of the interstitial space (Ve). These parameters can help distinguishing normal tissue from tumor tissue. DCE-MRI could have a role in the staging phase as it distinguishes histologic subtypes (Oberholzer et al. 2008). But it also perceives tumor microvascular density changes during chemoradiotherapy and can be imaged by DCE-MRI signal (Chang et al. 2008). Therefore, for monitoring response following CRT it holds the most promise.
2.3.5 Molecular Markers/Signatures
2.3.5.1 Background
Concurrent chemoradiation with or without surgery is commonly utilized as primary management of patients with non-metastatic disease. However, there is significant heterogeneity of response, suggesting that there are sub-populations that derive differential treatment benefit from RT. For example, approximately 30 % of patients experience a complete pathological response (Berger et al. 2005; Donahue et al. 2009). A molecular diagnostic that can identify these patients could be utilized clinically to avoid surgery for these patients. In contrast for patients that are predicted to be less responsive to RT, their management could be impacted by either offering RT dose intensification and/or prioritization of surgery (without RT).
At its most basic, a molecular signature is a collection of features that attempt to explain a complex phenotype. While a single predictive molecular marker would be ideal, such an isolated predictor of response to therapy in esophageal cancer has not been documented. In lieu of such a discovery, the technique of combining multiple analytes provides an opportunity to develop a predictive molecular assay; there continue to be a relatively small number of molecular signatures that are routinely part of clinical practice.
2.3.5.2 Molecular Signature Development
Developing a molecular signature typically involves two steps. In the first step, features (genes, proteins, microRNAs etc.) are selected that define the phenotype of interest (i.e. responders to radiation therapy). Once the features are selected then an algorithm is generated to predict the phenotype in an unknown sample. A classic approach is to use samples in a dataset as a “training set” to identify the features and develop the signature. Once the signature is developed, its predictive accuracy is tested on a validation set, ideally independent of the training set. A significant problem in the field of molecular signatures has been their inherent dependence on the “training set” and thus a lack of robust validation analysis (Watanabe et al. 2006; Dalton and Friend 2006).
2.3.5.3 Radiation Therapy Molecular Signatures
The majority of molecular signatures in the literature have been developed to describe disease prognosis (independent of treatment), molecular subtypes and/or response prediction to chemotherapy. However, two independent groups have developed RT-specific signatures that have considerable clinical validation. Weichselbaum and colleagues developed an interferon-related gene signature for DNA damage, which was independently validated as a predictor of adjuvant chemotherapy efficacy and for local–regional control after RT in breast cancer (Weichselbaum et al. 2008). Separately, Eschrich and colleagues utilized a systems biology approach to identify a molecular signature of intrinsic tumor radiosensitivity (Eschrich et al. 2009a, b). Using ten specific genes they modeled a radiosensitivity index (RSI) that has been independently validated in multiple disease sites (rectal, esophagus, head and neck, breast) in over 1,000 patients. Of the two signatures, RSI has been validated in a small dataset of esophageal cancer patients (n = 12). The predicted RSI was significantly different in responders (R) vs. nonresponders (NR) in esophageal (RSI R vs. NR 0.37 vs. 0.50, p = 0.05). A low RSI value is consistent with a more radiosensitive tumor. The range of RSI values for 7 responders was 0.11–0.53 and for 5 nonresponders was 0.46–0.54. Therefore it is possible that this signature can be adjusted to support specific clinical decisions to improve clinical care for esophageal cancer patients.
2.3.5.4 Clinical Applications for an RT Molecular Signature in Esophageal Cancer
A challenge to the development of a clinically relevant radiosensitivity molecular signature stems from RT’s broad applicability as a therapeutic agent in cancer. Since RT is used in different settings depending on disease site, the clinical utility of the signature would vary depending on the clinical application. A requirement for any signature that is to be applied routinely in the clinic is the development of a standardized and reliable process for tissue acquisition, processing, RNA isolation and gene expression measurement. Recently, the National Cancer Institute selected RSI for commercial development through the recently created Clinical Assay Development Program (CADP). The purpose of the project is the development of an analytically validated, commercial-grade diagnostic platform for RSI that will be ready for testing in clinical trials.
There remains significant opportunity to improve the clinical outcomes for esophageal cancer patients by identifying biological sub-populations that will derive differential treatment benefit from RT. Tailoring RT to fit a particular molecular RT profile will lead to the development of biology-based radiation oncology and result in better RT utilization.
3 Toxicity
The evolution of treatment for locally advanced esophageal cancer from single-modality surgery or radiotherapy to multimodality therapy has resulted in improved outcomes. Unfortunately, CRT comes with a potential increase in toxicity and resultant detriment to a patient’s short-term quality of life (van Meerten et al. 2008).
The risks vs. benefits of the treatment are decisive in patient’s decision to receive CRT. To make a well-considered decision, patients should be counseled before start of treatment about the potential toxicities. We give an overview of potential risks and toxicities for patients receiving multimodality treatment or single modality radiotherapy. We provide parameters, where available, that predict toxicity.
Among the many challenges with estimating toxicity risk based on the available publications, is that toxicity scoring systems are not uniform—making direct comparison impossible. The most frequently used scoring systems for toxicity are the Common Terminology Criteria for Adverse Events (CTCAE) (Trotti et al. 2003) of the National Cancer Institute (NCI) and the toxicity criteria from the Radiation Therapy Oncology Group (RTOG) system.
3.1 Esophagitis
The most common acute and late toxicity, excluding fatigue, for esophageal cancer patients treated with (chemo-) radiotherapy is, as expected, esophagitis.
3.1.1 Acute Esophagitis
Radiation induced acute esophagitis presents as dysphagia with resultant malnutrition and dehydration, requiring nutritional support (enteral or parenteral) in 17–35 % of the patients (Ahn et al. 2005).
The incidence of acute esophagitis (any grade) ranges from 19 to 79 % and grade ≥3 esophagitis is reported in 1–43 % of patients. This broad scale of the reported esophagitis depends, primarily, on the differences in toxicity reporting and definition. Other potential risk factors for esophagitis include dose schedules and treated volume of the esophagus. Finally, the incidence of esophagitis increases with the addition of chemotherapy to radiotherapy and may vary depending on the chemotherapy schedule given (Meluch et al. 2003; Urba et al. 2003; van Meerten et al. 2006; Cooper et al. 1999; Ajani et al. 2008).
3.1.2 Late Esophageal Toxicity
Late esophagitis can present as dysphagia, stricture, necrosis or fistula of the esophagus. The incidence rates of this late toxicity are mainly based on definitive chemoradiation studies. Late esophageal toxicity of any grade occurs in about 35 % of the patients and a grade ≥3 late esophageal toxicity has been seen in 8–21 % of the patients (Cooper et al. 1999).
The strongest predictor for late esophageal toxicity is the severity of acute esophagitis, as a result of consequential late effects. Other predicting parameters for esophageal toxicity are dosimetric.
3.1.3 Parameters Predicting Esophagitis
From lung cancer series we have learned several predicting parameters for acute and late esophageal toxicity. As described earlier, the strongest parameter is the severity of the acute toxicity, but other parameters are a combination of radiation dose and treated volume of the esophagus.
A number of dosimetric parameters have been developed in an effort to reduce the continuously distributed dose-volume histogram (DVH) to a few clinically relevant indices. These relevant indices include: the percent organ volume receiving at least a certain dose (V20Gy, V30Gy, V40Gy); the surface area receiving at least a certain dose (SA20Gy, SA30Gy, SA40Gy); the length of the esophagus included in the radiation field to a threshold dose (LETT20Gy, LETT30Gy, LETT40Gy); the mean esophageal dose (MED), defined as the average dose to the esophagus; and the maximal dose, defined as the highest point-dose within the irradiated esophageal volume (Milano et al. 2007; Rose et al. 2009).