Stage
Descriptions
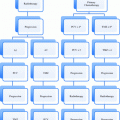
Primary Tumor (T)
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Tis
Carcinoma in situ
T1
Tumor limited to the pancreas, 2 cm or less in greatest dimension (resectable primary tumor)
T2
Tumor limited to the pancreas, more than 2 cm in greatest dimension (resectable primary tumor)
T3
Tumor extends beyond the pancreas but without involvement of the celiac axis or superior mesenteric artery (potentially resectable primary tumor)
T4
Tumor involves the celiac axis or the superior mesenteric artery (unresectable primary tumor)
Regional Lymph Nodes (N)
NX
Regional lymph nodes cannot be assessed
N0
No regional lymph node metastasis
N1
Regional lymph node metastasis.
Regional lymph node sampling from nodes around common hepatic artery, celiac artery, splenic hilum, infrapyloric nodes are required during Whipple’s procedure in pathological staging. Ideally, >10 lymph nodes should be sampled during surgery
Distant Metastasis (M)
MX
Distant metastasis cannot be assessed
M0
No distant metastasis
M1
Distant metastasis (including seeding of the peritoneum and positive peritoneal cytology)
Stage Grouping
T1
T2
T3
T4
N0 IA
IB
IIA
III
N1 11B
IIB
IIB
III
Ml IV
IV
IV
IV
3 Multidisciplinary Management of Resectable Tumors
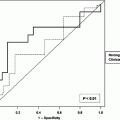
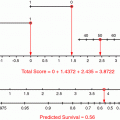
Multiple studies have demonstrated that clinicopathologic factors such as tumor size, histologic differentiation, margin status, and nodal involvement are statistically significant prognostic variables (Corsini et al. 2008; Miller et al. 2009). The pancreatic nomogram, originally developed in the Memorial Sloan-Kettering Cancer Center (MSKCC) in the USA, combines clinicopathological and operative data to predict disease-specific survival at 1, 2 and 3 years from initial resection. It was based on prospectively collected data from 555 pancreatic resections for adenocarcinoma (Brennan et al. 2004). Factors include age, gender, weight loss, T stage, number of positive and negative nodes, differentiation, margins and others. An external patient cohort from a retrospective pancreatic adenocarcinoma database at the Academic Medical Centre in Amsterdam was used to test the validity of the nomogram (De Castro et al. 2009). The cohort included 263 consecutive patients who had surgery between 1985 and 2004. The 1-, 2- and 3-year disease-specific survival rates were 61, 30 and 16 % respectively. The nomogram concordance index was 0.61. The calibration analysis of the model showed that the predicted survival did not significantly deviate from the actual survival. Thus, this model may aid in counseling patients.
Historically, chemoradiation has been shown to reduce the probability of local tumor recurrence (Anonymous 1987). Chemoradiation accomplishes this by eradicating microscopic residual disease remaining in the tumor bed after complete tumor resection or through the reduction in regional lymph node recurrence (Belka et al. 2006). In the case of pancreatic cancer, the retroperitoneal margin is nearly always close and often positive, and isolated lymph node recurrences are rare. Therefore, at least in theory, locoregional therapy in pancreatic cancer can be optimized with complete gross tumor resection and treatment of microscopic disease at the retroperitoneal margin with chemoradiation. Unfortunately, however, multiinstitutional trials have reported strikingly high rates of local tumor recurrence. Local tumor recurrence (or more likely) persistence was identified as a component of the first site of failure in 39 % of patients enrolled on the GITSG trial (Anonymous 1987), 53 % of patients enrolled on the EORTC trial (Klinkenbijl et al. 1999), and 62 % of patients enrolled on the ESPAC-1 trial (Neoptolemos et al. 2004). Moreover, several trials questioned whether chemoradiation truly improves survival more than adjuvant chemotherapy. Schmidt et al. reported a randomized phase III trial of adjuvant chemoradiation plus interferon alfa-2b versus fluorouracil (5-FU) and folinic acid (FA) for patients with resected pancreatic adenocarcinoma (Schmidt et al. 2012). Between 2004 and 2007, 132 R0/R1 resected patients received either 5-FU, cisplatin, and interferon alfa-2b plus radiotherapy followed by two cycles of 5-FU (arm A, n = 64) or six cycles of 5-FU monotherapy (arm B, n = 68). Median survival for all randomly assigned patients was 26.5 months in arm A and 28.5 months in arm B. The hazard ratio was 1.04, p = 0.99. A randomized phase II intergroup study explored the feasibility and tolerability of a gemcitabine-based regimen after R0 resection of pancreatic head cancer (Van Laethem et al. 2010). Patients (n = 90) were randomly assigned to receive either four cycles of gemcitabine (control arm) or gemcitabine for two cycles followed by weekly gemcitabine with concurrent radiation (50.4 Gy; CRT arm). Treatment was completed per protocol by 87 and 73 % in the control and CRT arms, respectively, and grade 4 toxicity was 0 and 5 %, respectively. In the CRT arm, three patients experienced grade 3-related late toxicity. Median DFS was 12 months in the CRT arm and 11 months in the control arm. Median OS was 24 months in both arms. First local recurrence was less frequent in the CRT arm (11 % vs. 24 %).
As compared to Europe, adjuvant chemoradiation is more commonly used in the United States (Kimple et al. 2012; Van Laethem et al. 2012). For adjuvant therapy, the Radiation Therapy Oncology Group (RTOG) has defined in a consensus panel guidelines for the delineation of the clinical target volume (CTV) in pancreatic head cancer (Goodman et al. 2012). In addition to the preoperative tumor volume and the pancreaticojejunostomy these guidelines define the following elective nodal volumes: celiac artery, superior mesenteric artery, portal vein and aorta. One of their landmark clinical trials was RTOG 9704 (Willett et al. 2003; Regine et al. 2011). After resection of pancreatic adenocarcinoma, patients were randomized to pre- and post-chemoradiation 5-FU versus pre- and post-chemoradiation gemcitabine. CRT was provided at 50.4 Gy with continuously provided 5-FU. Four hundred fifty-one patients were eligible. Univariate analysis showed no difference in overall survival (OS). Pancreatic head tumor patients (n = 388) had a median survival and 5-year OS of 20.5 months and 22 % with gemcitabine versus 17.1 months and 18 % with 5-FU. On multivariate analysis, patients on the gemcitabine arm with pancreatic head tumors experienced a trend toward improved OS (p = 0.08). The sequencing of 5-FU CRT with gemcitabine as done in this trial was not associated with a statistically significant improvement in OS. Despite local recurrence being approximately half of that reported in previous adjuvant trials, distant disease relapse still occurred in more than 70 % of patients. These findings served as the basis for the ongoing EORTC/U.S. Intergroup RTOG 0848 phase III adjuvant trial evaluating the impact of CRT after completion of a full course of gemcitabine. RTOG 9704 data were also used to determine the influence of lymph node factors (number of positive nodes (NPN), total nodes examined (TNE), and lymph node ratio (LNR ratio of NPN to TNE)) on OS and disease-free survival (DFS). Both TNE, NPN, and LNR were associated with OS and DFS (Showalter et al. 2011). A previous multivariate analysis of RTOG 9704 demonstrated two significant factors that predicted OS: nodal involvement (hazard ratio 1.5) and CA 19-9 level >90 (hazard ratio 3.3) (Berger et al. 2008). CA 19-9 is a stratification factor for the current RTOG adjuvant pancreas trial (0848) (Table 2).
Table 2
Major prognostic factors in pancreatic adenocarcinoma
Resectable stages | Locally advanced, non-metastatic disease |
|---|---|
Tumor size | Performance status |
Tumor location (head versus other) | Age |
Nodal involvement | CA 19-9 level |
Histologic differentiation | Uncertainty regarding distant metastases (stage Mx) |
CA 19-9 level | PET-CT related features such as SUVmax and tumor volume |
Better prognosis is also expected in patients with younger age, less weight loss and female gender |
A different North American study was reported by Moghanaki et al. (2011). Between 1984 and 2006, this group retrospectively analyzed 91 patients with pancreatic cancer treated with pancreaticoduodenectomy or total pancreatectomy followed by adjuvant 5-FU-based chemoradiation at the University of Pennsylvania. The prognostic significance of demographic factors, stage, year of surgery, tumor location, grade, resection status, and number of positive lymph nodes on overall survival were examined. With a median follow-up of 6.5 years, the overall median survival was 2.3 years, and the 5-year overall survival was 29 %. In multivariate analysis, completeness of resection (p<0.001), fewer number of positive lymph nodes (0 vs. 1–2 vs. 3 or more) (p = 0.004), and age ≤60 years (p = 0.006) were all independently associated with improved overall survival. Some centers have used intraoperative radiotherapy (IORT) with or without external beam radiotherapy (EBRT) and chemotherapy (Pisters et al. 1998; Jingu et al. 2012). However, the focus of this chapter is not on technical aspects of radiotherapy.
4 Down-Staging Patients with Locally Advanced Disease
There is a widespread perception that some unresectable pancreatic tumors can be converted to resectable ones with the use of chemotherapy or chemoradiation. The interpretation of published studies is limited by inconsistent and subjective definitions of resectability and by variable, in part inadequate preoperative radiologic assessments of resectability. Probably the most variable factor in determining resectability and thus interpreting whether a tumor has been converted to resectable from unresectable is the meaning of vascular involvement (Katz et al. 2013). Although most surgeons would agree that tumor encasement of either the celiac artery or the superior mesenteric artery constitutes unresectable disease, opinions vary with regard to more limited arterial involvement. It is probably in this group of patients that, theoretically, active cytotoxic therapy could lead to down-staging (Golcher et al. 2008). These cases are sometimes referred to as “marginally resectable” or borderline resectable. In a recent study, patients were deemed borderline resectable if they had severe unilateral superior mesenteric vein or portal vein impingement, tumor abutment of the superior mesenteric artery, gastroduodenal artery encasement up to the origin from the hepatic artery, or colon invasion (Kim et al. 2013). Sixty-eight evaluable patients received treatment at 4 centers. Treatment consisted of two 28-day cycles of gemcitabine (1,000 mg/m2 on days 1, 8, and 15) and oxaliplatin (85 mg/m2 on days 1 and 15) with radiotherapy during cycle 1 (30 Gy in 2-Gy fractions). Patients were evaluated for surgery after cycle 2. Patients who underwent resection received 2 cycles of adjuvant chemotherapy. The only characteristic associated significantly with R0 resection was CA 19-9 response. Comparing any increase (n = 15) with a 0–50 % decrease (n = 13) and a >50 % decrease (n = 27) demonstrated that a decrease in CA 19-9 was associated with R0 resection (p = 0.02). Resection (R0 vs R1/R2 vs none), baseline quality of life, female gender, tumor in the pancreatic body or tail (compared with the pancreatic head), and lower CA 19-9 levels at baseline were associated with improved survival (all p < 0.05). In the patients who underwent surgical resection (n = 43), longer surgery time (p = 0.03) and increased blood loss during surgery (p = 0.02) were associated with poorer survival, and marginal associations were observed with surgical procedure (Whipple was inferior to distal-subtotal pancreatectomy; p = 0.057) and histologic treatment effect (p = 0.068).
In Heidelberg, Germany, a total of 215 patients with locally advanced pancreatic cancer were treated with chemoradiation at a single institution (Habermehl et al. 2012). Radiotherapy was delivered with a median dose of 52.2 Gy in single fractions of 1.8 Gy. Chemotherapy was applied concomitantly as gemcitabine at a dose of 300 mg/m2 weekly, followed by adjuvant cycles of full-dose gemcitabine (1,000 mg/m2). After neoadjuvant treatment restaging was done to evaluate secondary resectability. After chemoradiation a total of 26 % of all patients with primary unresectable disease were offered secondary resection. Tumor-free resection margins could be achieved in 39 % (R0-resection). Patients with complete resection after CRT showed a significantly increased median overall survival with 22.1 compared to 11.9 months in non-resected patients. In most cases the first site of disease progression was systemic with hepatic (52 %) and peritoneal (36 %) metastases. A different study population from the United States was comprised of 240 consecutive patients who received neoadjuvant chemoradiation and surgery, and was compared with 60 patients who had no neoadjuvant therapy between 1999 and 2007 (Estrella et al. 2012). Among the 240 treated patients, the 1-year and 3-year DFS rates were 52 and 32 %, with a median DFS of 15.1 months. The 1-year and 3-year OS rates were 95 and 47 %, with a median OS of 33.5 months. By univariate analysis, DFS was associated with age, post-therapy tumor stage (ypT), lymph node status (ypN), number of positive lymph nodes, and AJCC stage, whereas OS was associated with intraoperative blood loss, margin status, ypT, ypN, number of positive lymph nodes, and AJCC stage. By multivariate analysis, DFS was independently associated with age, number of positive lymph nodes, and AJCC stage, and OS was independently associated with differentiation, margin status, number of positive lymph nodes, and AJCC stage. In addition, the treated patients had better OS and lower frequency of lymph node metastasis than those who had no neoadjuvant therapy. In a series of 132 North American patients (Breslin et al. 2001), survival duration was superior for women (p = 0.04) and for patients with no evidence of lymph node metastasis (p = 0.03). There was no difference in survival duration associated with patient age, dose of preoperative radiation therapy, the delivery of intraoperative radiotherapy, or the histologic grade of chemoradiation treatment effect.
Currently, stereotactic body radiotherapy (SBRT) is also being explored in comparable patient groups (Polistina et al. 2010; Chuong et al. 2013). Approximately one-third of initially staged non-resectable tumor patients would be expected to have resectable tumors following neoadjuvant therapy, with comparable survival as initially resectable tumor patients (Gillen et al. 2010). A recent meta-analysis included 19 studies, which involved 2,148 patients (Laurence et al. 2011). Only cohort studies were included. The meta-analysis found that patients with unrespectable pancreatic cancer who underwent neoadjuvant chemoradiotherapy achieved similar survival outcomes to patients with resectable disease, even though only 40 % were ultimately resected. Neoadjuvant chemoradiotherapy was not associated with statistically significant increase in the rate of pancreatic fistula formation or total complications, although there was an increase in the risk of peri-operative death.
White et al. performed a study to evaluate the applicability of an already mentioned nomogram, which was developed for patients undergoing resection without preoperative chemoradiation and which incorporates several post-resection pathological factors (Brennan et al. 2004), to a population of patients who received preoperative CRT prior to resection (White et al. 2006). From 1994 to 2004, 82 patients with biopsy-proven, radiographically localized adenocarcinoma of the pancreatic head underwent preop CRT followed by pancreaticoduodenectomy (PD); 50 concurrent patients underwent PD without preoperative CRT. Mean nomogram-predicted disease-specific survival rates were compared with observed rates from the time of resection. Despite having more locally advanced tumors on initial staging (21 vs. 8 %; p<0.05), patients who received preoperative CRT had smaller resected tumors (mean 2.3 vs. 3.1 cm; p<0.01), were less likely to have T3 tumors (54 vs. 80 %, p<0.01), were less likely to have positive lymph nodes (29 vs. 58 %, p< 0.01), and had fewer positive lymph nodes (mean 0.4 vs. 1.9, p<0.01), all factors that imply treatment effect and favorably impact on nomogram-predicted outcome. Observed DSS was similar to predicted DSS in both groups. The similarity in observed and predicted DSS following resection in patients who received preoperative CRT suggested that the effects (whether treatment, selection, or no effect) were reflected by the nomogram. The ability of the nomogram to evaluate the effects of preoperative CRT on survival was limited by the potential effects of preoperative CRT on factors within the nomogram.
5 Chemoradiation in Locally Advanced Disease (LAPC)
Locally advanced pancreatic cancer is generally incurable and all therapies have significant limitations. In reality, patients probably benefit modestly from both systemic therapy and chemoradiation; these approaches are complementary and should both be considered in patients with locally advanced disease (Huguet et al. 2007). Acute toxicity can be reduced if the radiation fields are confined to the gross primary tumor and clinically enlarged lymph nodes, regardless of the radiosensitizing chemotherapy that is used (Jackson et al. 2010). Treating uninvolved regional lymph nodes requires larger amounts of gastric and duodenal mucosa to be treated which can lead to higher rates of gastrointestinal toxicity and is not likely to improve median survival.
In GITSG studies of 5-FU–based chemoradiation therapy in patients with locally advanced pancreatic adenocarcinoma (Anonymous 1979; Moertel et al. 1981) patients were randomly assigned to receive 40 Gy of radiation plus 5-FU, 60 Gy plus 5-FU, or 60 Gy without chemotherapy. Radiation therapy was delivered as a split course, with 20 Gy given over 2 weeks followed by a 2-week rest. 5-FU was delivered IV at a bolus dose of 500 mg/m2/day for the first 3 days of each 20-Gy cycle and given weekly (500 mg/m2) following the completion of chemoradiation therapy. The median survival was 10 months in each of the chemoradiation groups and 6 months for the group that received 60 Gy without 5-FU. In contrast to the results from the GITSG, an ECOG study suggested no benefit to chemoradiation therapy over 5-FU alone (Klaassen et al. 1985). The ECOG study randomly assigned patients with locally advanced or incompletely resected pancreatic adenocarcinoma to receive chemoradiation therapy (40 Gy and 600 mg/m2/day 5-FU for 3 days) or 5-FU alone (600 mg/m2/week). The chemoradiation therapy group received weekly bolus administration of 5-FU following chemoradiation therapy until there was evidence of disease progression. The median survival was 8.3 months in the group that received chemoradiation therapy and 8.2 months in the group that received 5-FU alone. It was evident both in the GITSG studies and in the ECOG trial that patients with locally advanced, unresectable pancreatic cancer who are symptomatic to the point of not being fully ambulatory do not benefit from anticancer therapy. More recent trials of chemoradiation for locally advanced pancreatic cancer have investigated continuous-infusion 5-FU in combination with EBRT. Capecitabine appears to have similar efficacy to intravenously administered 5-FU and is an appropriate substitute for infusional or bolus 5-FU when used with radiotherapy. In a phase I trial, 800 mg/m2 was the recommended dose when capecitabine was given on days of radiation only (Saif et al. 2005).
The introduction of gemcitabine was a step forward in the treatment of pancreatic cancer. Its value as a systemic agent and the recognition of its radiosensitizing properties stimulated the study of combinations of gemcitabine with EBRT for patients with localized pancreatic cancer (Blackstock et al. 1999; McGinn et al. 2001; Pipas et al. 2001; Wolff et al. 2001; Murphy et al. 2007; Girard et al. 2010; Brunner et al. 2011; Cardenes et al. 2011). Several strategies have been investigated including seven-weekly injections of gemcitabine with short course EBRT (30 Gy), twice-weekly gemcitabine with 50.4 Gy of EBRT, weekly gemcitabine with 50.4 Gy of EBRT, and full dose weekly gemcitabine with escalating doses of radiation. Most of these studies suggested gastrointestinal toxicity as a dose-limiting factor, but hematologic toxicity has also been observed. Several multiinstitutional studies have been completed evaluating gemcitabine-based chemoradiation. In a small study performed in Taiwan, 34 patients with locally advanced pancreatic cancer were randomized to receive 5-FU based chemoradiation (500 mg/m2 daily for 3 days, every 14 days with radiation to a total dose of 50.4–61.2 Gy) or gemcitabine and radiation (600 mg/m2 weekly with equivalent doses of radiation) (Li et al. 2003). The objective response rate to gemcitabine and radiation was 50 % and only 13 % for 5-FU chemoradiation. In addition, median survival was substantially better using gemcitabine compared with 5-FU (14.5 months vs. 6.7 months, p = 0.027). These efficacy results must be interpreted with caution because of the limited accrual (34 patients) and the poor results in the control group. A phase II study conducted in patients with locally advanced pancreatic cancer by the Cancer and Leukemia Group B evaluated gemcitabine given at 40 mg/m2 twice weekly. In that study, there were 35 and 50 % grade 3 or 4 gastrointestinal and hematologic toxicities, respectively, and the median survival was only 8.5 months (Blackstock et al. 2001) Not surprisingly, the Cancer and Leukemia Group B abandoned this approach in locally advanced pancreatic cancer. Both of these studies used regional nodal fields that likely contributed to the significant gastrointestinal toxicity. In contrast, the approach that was developed at the University of Michigan delivered high doses of gemcitabine (1,000 mg/m2) and a slightly lower radiotherapy dose (36 Gy in 15 fractions over 3 weeks), with conformal radiation fields encompassing the gross tumor volume alone. At that institution, the irradiation of a smaller volume of normal tissue was reported to be well tolerated (McGinn et al. 2001). Investigators have since embarked on further studies evaluating the same regimen. As reported in 2008, 41 patients enrolled at six institutions (Small et al. 2008




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree