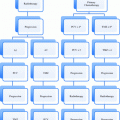
T—primary tumor
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
T1
Clinically unapparent tumor not palpable or visible by imaging
T1a
Tumor incidental histological finding in 5 % or less of tissues resected
T1b
Tumor incidental histological finding in more than 5 % of tissue resected
T1c
Tumor identified by needle biopsy (e.g. because of elevated PSA level)
T2
Tumor confined within the prostate
T2a
Tumor involves one half of one lobe or less
T2b
Tumor involves more than half of one lobe, but not both lobes
T2c
Tumor involves both lobes
T3
Tumor extends through the prostate capsule
T3a
Extracapsular extension (unilateral or bilateral)
T3b
Tumor invades seminal vesicle(s)
T4
Tumor is fixed or invades adjacent structures other than seminal vesicles. External sphincter, rectum, levator ani and/or pelvic wall
N—regional lymph nodes
NX
Regional lymph nodes cannot be assessed
N0
No regional lymph node metastasis
N1
Regional lymph node metastasis
M—distant metastasis
M0
No distant metastasis
M1
Distant metastasis
M1a
Non-regional lymph node(s)
M1b
Bone(s)
M1c
Other site(s)
Beyond Gleason score, pretreatment serum PSA, and stage at diagnosis, additional pathologic factors including percent positive biopsy cores, PSA density and velocity, length of core involvement by tumor, and presence of perineural invasion also portend prognostic significance.
Since the clinical behavior of prostate cancer might range from indolent to highly aggressive, prognostic assessment is important for predicting outcome and treatment selection. A number of prognostic schemes have been developed. The clinical grouping system developed by the National Comprehensive Cancer Network (NCCN) is summarized, in addition with suggested general risk-adapted treatment recommendations in Table 2. Localized prostate cancer can be treated with surgery, radiation therapy, or the combination of both. In addition, hormonal therapy plays a role in the treatment of locally advanced disease. For selected patients with very low- or low-risk disease, active surveillance may be a valid option. It should be noted that no randomized trial exists comparing modern radiation with prostatectomy techniques, and that oncologic outcomes for localized prostate cancer appear similar, when appropriate radiation doses are employed (Kupelian et al. 2004). In a recent large scale comprehensive review of the literature by Grimm et al. (2012) comparing risk stratified patients by treatment option and with long-term follow-up, the statistical analysis suggested that, in terms of biochemical-free progression, brachytherapy provides superior outcome in patients with low-risk disease. For intermediate-risk disease, the combination of EBRT and brachytherapy appears equivalent to brachytherapy alone. For high-risk patients combination therapies involving EBRT and brachytherapy plus or minus androgen deprivation therapy appear superior to more localized treatments such as seed implant alone, surgery alone or EBRT.
Risk group | Parameter | Suggested treatment strategy |
|---|---|---|
Very low | T1a, Gleason ≤ 6, PSA < 10, <3 positive biopsy cores and <50 % cancer per core | Active surveillance using PSA and DRE, if expected survival <20 years |
Low | T1–T2a, Gleason ≤ 6 and PSA < 10 | Active surveillance using PSA and DRE if expected survival <10 years, using PSA, DRE, and repeat biopsy if expected survival ≥10 years, or definitive therapy using IG-IMRT, brachytherapy, or radical prostatectomy (RP) |
Intermediate | T2b–T2c, Gleason 7, or PSA 10–20 | IG-IMRT with or without short-term ADT with or without brachytherapy boost or RP |
High | T3a, Gleason 8–10, or PSA > 20 | IG-IMRT and long-term ADT or radical prostatectomy plus PLND with or without adjuvant RT |
Locally advanced: very high | T3b–T4 | IG-IMRT plus long-term ADT or radical prostatectomy plus PLND with or without adjuvant RT or ADT |
Locally advanced: LN | N1 | ADT or IG-IMRT and long-term ADT |
Distant metastases | M1 | ADT |
2 Treatment of Non-Metastatic Prostate Cancer
External-beam radiation therapy (EBRT) is one of the most important definitive treatment modalities for localized prostate cancer of all stages. Following initial single-institution reports of improved efficacy and therapeutic ratio by Hanks et al. (1998), dose-escalation strategies have become an important focus of prostate cancer research endeavors. Clinical evidence for dose escalation in EBRT is presented in Table 3 for randomized clinical trials.
Table 3
Main randomized studies on localized PCa supporting dose escalation
Reference | Number of patients | Dose escalation | Endpoint | Comment |
|---|---|---|---|---|
Kuban et al. 2008 | 301 | 78 versus 70 Gy | ↑ 8-year biochem. DFS: 78 versus 59 % | Largest benefit among patients with pretreatment PSA > 10 ng/ml |
Peeters et al. 2006 | 669 | 78 versus 68 Gy | 5-year FFF sign better after 78 Gy: 64 versus 54 % | No sign differences in FFCF or OS |
Zietman et al. 2010 | 393 | 70.2 or 79.2 GyE proton RT | 10-year ASTRO BF 32.4 versus 16.7 % for high-dose RT | Difference was largely due to low- and intermediate disease |
Several randomized and non-randomized studies have shown that dose escalation with a dose-range of 76–80 Gy has a significant impact on 5-year survival without biochemical relapse. Two randomized trials focused on clinical stages T1-3 N0 M0 and opened the clinical decision for dose escalation. The MD Anderson study (Kuban et al. 2008) compared 78 with 70 Gy conventional radiotherapy. It included 305 patients stage T1b to T3 with a median follow-up of 8.7 years. The results showed a significant increase in freedom from biochemical and/or clinical failure (p = 0.004), which was largest for patients with initial PSA > 10 ng/ml (p = 0.001) (Table 3).
The PROG 95-09 study (Zietman et al. 2010) evaluated 393 T1b-T2b patients, of whom 75 % had a Gleason score < 6 and a PSA < 15 ng/ml. Patients were randomized to receive an initial boost to the prostate alone, using conformal protons of either 19.8 or 28.8 Gy, and then 50.4 Gy to a larger volume. With a median follow-up of 5.5 years, there was a significant increase in 5-year freedom from biochemical failure (p < 0.001) in favour of low-risk patients given a higher dose (79.2 Gy) versus those given a conventional dose (70.2 Gy). There was a strong trend in the same direction for the intermediated-risk patients (n = 144, p = 0.06). 11 versus 6 % of patients subsequently required ADT for recurrence after conventional versus high-dose RT (p = 0.047). There remains no difference in OS (78.4 vs. 83.4 %, p = 0.41) and toxicity rates (1–3 % of grade 3–4) between the two arms.
A Dutch randomized phase III trial (Peeters et al. 2006) comparing 68 with 78 Gy showed a significant increase in 5-year freedom from clinical or biochemical failure (FFF or FFCF) for patients in an intermediate risk-group. 669 patients with T1b–T4 diseases were enrolled. With a follow-up of 51 months, 5-year FFF was significantly better after 78 Gy (64 vs. 54 %). No difference in late genitourinary or gastrointestinal toxicity was observed. As a result of these randomized studies, a minimum dose >74 Gy is recommended for EBRT.
Androgen-deprivation therapy (ADT), consisting of a combination of luteinizing-hormone-releasing hormone (LHRH) suppression and an anti-androgen, has been evaluated as an adjunct to standard-dose EBRT as an alternative strategy to improve outcomes in patients with intermediate- and high-risk prostate cancer. The supportive trials, although heterogeneous in their patient selection criteria and radiation treatment volumes, generally demonstrate statistically and clinically significant improvements in overall survival. Evidence in support of androgen suppression is most mature in patients with high-risk disease. Beginning in the 1980s research organizations in the USA and Europe launched a series of trials for which mature follow-up data are now available, and which have systematically evaluated the efficacy, timing and duration of androgen suppression therapy. For overview of studies see Table 4.
Table 4
Main trials combining RT plus hormonal treatment (intermediate-risk prostate cancer)
Reference | Number of patients | Treatment schedule | Endpoint | Comment |
|---|---|---|---|---|
D’Amico et al. 2008 | 206 | 70.35 Gy in 36 fractions +6 months of ADT | All-cause mortality was sign greater in RT alone arm | Sign difference was primarily in patients with no or minimal comorbid illness |
Denham et al. 2008 | 802 | RT to 66 Gy alone or RT plus 3 or 6 months of ADT before and during RT | Sign improvement in PCa-specific mortality with 6 but not 3 months of ADT |
Two randomized trials demonstrated clinical evidence on combined EBRT with hormonal therapy for intermediate-risk prostate cancer. D’Amico et al. reported on a phase III clinical trial comparing RT with or without 6 months of ADT. 80 % of 206 randomized patients had intermediate-risk prostate cancer (D’Amico et al. 2004, 2008). Conformal radiation comprised 70.35 Gy in 36 fractions prescribed to the prostate and seminal vesicles with a cone-down boost to the prostate. Initial results at a median follow-up of 4.5 years revealed statistical improvements in prostate cancer-specific survival, survival free of salvage androgen deprivation, and OS rate. Updated results after a median follow-up of 7.6 years showed that all-cause mortality was significantly greater in the RT alone arm (HR 1.8, p = 0.01). Subgroup analysis suggested that the significant difference was primarily in patients with no or minimal comorbid illness.
The TROG 96.01 randomized clinical trial (Trans-Tasman Radiation Oncology Group, Australia) studied the optimal duration of short-course hormonal therapy (Denham et al. 2008). This three-arm study compared prostate-only radiation to 66 Gy, versus radiation and 3 or 6 months of androgen deprivation before and during radiation for intermediate-risk patients. Results revealed a significant improvement in prostate-cancer-specific mortality with 6 (HR 0.56) but not 3 (HR 0.95) months of short-term androgen deprivation.
Fortunately, the incidence of locally advanced PCa (T3-4N0M0) has declined as a result of individual or mass screening. Pelvic lymph node irradiation is optional (see Sect. 3), but the results of radiotherapy alone are unsatisfactory. Because of the hormonal dependence of PCa, ADT has been combined with external irradiation in locally advanced PCa (T3-4N0M0) with the aim of reducing the risk of distant metastasis and decreasing the risk of non-sterilisation and/or local recurrence as a source of secondary metastases. Numerous randomized trials have confirmed the value of long-term administration.
The RTOG study 86-10 (Pilepich et al. 2001) included 471 patients with bulky (>5 × 5 cm) tumours T2-4 N0-X M0. Androgen deprivation therapy was administered at 2 months before irradiation and during irradiation, or in the case of relapse in the control arm. 32 % of patients were diagnosed as T2, 70 % as T3-4, and 91 % as N0. The hormone treatment consisted of oral eulexine, 250 mg three times daily, and goserelin acetate (Zoladex) 3.6 mg every 4 weeks by subcutaneous injection. RT target volumes and doses were similar to RTOG 85-31 (Pilepich et al. 1997; Lawton et al. 2001), also including the regional lymphatics to an initial 44–46 Gy, followed by a prostate boost to 65–70 Gy. The 10-year overall survival estimates were 43 % for ADT plus irradiation versus 34 % for hormonal treatment, although the difference was not significant (p = 0.12). There was a significant improvement in the 10-year disease-specific mortaliy (23 vs. 36 %; p = 0.01), DFS (11 vs. 3 %; p < 0.0001) and in biochemical failure (65 vs. 80 %; p < 0.0001). No significant impact on the risk of fatal cardiac events was seen with the addition of ADT.
The RTOG 85-31 trial (Pilepich et al. 1997; Pilepich et al. 2005; Lawton et al. 2001) randomized 977 patients to adjuvant goserelin (Arm I) versus observation (Arm II) with hormones initiated at relapse. Eligible patients had advanced tumor characteristics (T3-4 N0-1 M0) or pathologic penetration (pT3) through the capsule to the resection margin or seminal vesicle involvement after RP. Androgen deprivation therapy was begun in the last week of irradiation and continued up to relapse or was started at recurrence. A total of 15 % of patients in the first group and 29 % in the second group had undergone RP, 14 and 26 % were pN1. Goserelin was administered every 4 weeks. RT portals included treatment of the regional lymphatics to an initial 44–46 Gy (except in node-negative postoperative cases) followed by a prostate boost to 65–70 Gy. With a median follow-up of 7.6 years for all patients, at 10 years, the absolute survival rate was significantly greater for the adjuvant arm than for the control arm: 49 versus 39 % (p = 0.002). The 10-year local failure rate for the adjuvant arm was 23 versus 38 % for the control arm (p < 0.0001). The corresponding 10-year rates for the incidence of distant metastases and disease-specific mortality was 24 versus 39 % (p < 0.001) and 16 versus 22 % (p = 0.0052), respectively, both in favour of the adjuvant arm (Lawton et al. 2001, 2008; Pilepich et al. 2005).
The EORTC 22863 was an open-labeled randomized phase 3 trial (Bolla et al. 2002, 2010). Eligible patients were younger than 80 years and had newly diagnosed histologically proven T1–2 prostatic adenocarcinoma with WHO histological grade 3, or T3-4N0M0 and any histological grade. The trial compared EBRT and adjuvant long-term (concurrent and adjuvant) androgen suppression with radiotherapy alone. EBRT initially targeted the prostate and pelvic nodes to 50 Gy, with a subsequent prostate-only boost to an additional 20 Gy. Hormonal therapy consisted of monthly goserelin administration for 3 years, beginning on the first day of EBRT and 1 month of cyproterone acetate. With a median follow-up of 66 months, combination therapy compared with radiotherapy alone yielded significantly better survival (78 % vs. 62 %, p = 0.001) (Bolla et al. 2002). At a median follow-up of 9.1 years, the 10-year overall survival remained significantly higher at 58.1 versus 39.8 % (p < 0.0001), as did clinical progression-free survival at 47.7 versus 22.7 % (p < 0.0001). The 10-year cumulative incidence of PCa mortality was 11 versus 31 % (p < 0.0001). No significant difference in cardiovascular mortality was noted between treatment groups both in patients who had cardiovascular problems at study entry and in those who did not. The 10-year cumulative incidence of cardiovascular mortality was 11.1 versus 8.2 % (p = 0.75) (Bolla et al. 2010).
In conclusion the trials devoted to locally advanced PCa have shown a significant gain in overall survival of the combination of EBRT and long-term ADT and raised the question of whether the gain was due to ADT alone rather than to the combined approach. However, many trials were launched to assess the value of a long-term ADT plus or minus irradiation.
Mottet et al. (2012) report on the results of a phase 3 multicentric randomized trial devoted to 264 N0-X patients classified as cT3–4 (n = 254) or pT2 with positive biopsies of the capsule (n = 10), randomly allocated between long-term (3-year) ADT alone or combined with three-dimensional conformal radiotherapy (3D-CRT). ADT was administered with an LHRH-agonist (leuprorelin) given subcutaneously with a 3-monthly depot and an oral antiandrogen (flutamide) for 1 month to inhibit flare-up. In the ADT alone arm, 33 patients received salvage RT for local progression. RT was focused on the pelvis with a four-field box technique (46 +




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree