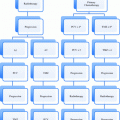
Histological type
Remarks
Treatment strategy
Urothelial cancer, transitional cancer
Most frequent subtype (> 90–95 %)
Superficial tumors
TUR ± intravesical therapy is standard
Radiochemotherapy indicated, if otherwise cystectomy would be performed
Cystectomy for recurrent and progressive tumors after bladder-sparing initial treatment
Immediate cystectomy as option in high-risk tumors (T1 G3)
Muscle-invasive tumors (T2-4)
Organ-preservation with TUR and radiochemotherapy, salvage-cystectomy only in case of relapse
Radical cystectomy, (neo)adjuvant chemotherapy for high-risk patients
Squamous cell cancer
Rare (about 5 %), mostly advanced, prognosis slightly inferior to urothelial cancer
Few data. Treatment as muscle-invasive urothelial cancer is recommended
Adenocarcinoma
Rare (<1 %)
Surgery (partial or complete cystectomy) is standard. No data on efficacy of radiotherapy
Undifferentiated small cell cancer
Very rare (≪1 %)
Treatment as extrapulmonary small cell cancer
1.3 TNM Classification
The Tumor, Node, Metastasis (TNM) Classification of Malignant Tumors is the method most widely used to classify the extent of cancer spread (Table 2). Concerning histological grading, the use of the 2004 WHO classification is recommended but still needs to be validated by more clinical trials. Most clinical trials published so far on bladder tumors have been performed using the 1973 WHO Classification.
Table 2
2009 TNM classification of urinary bladder cancer (updated 2012)
T: Primary tumor |
TX Primary tumor cannot be assessed |
T0 No evidence of primary tumor |
Ta Non-invasive papillary carcinoma |
Tis Carcinoma in situ: ‘flat tumor’ |
T1 Tumor invades subepithelial connective tissue |
T2 Tumor invades muscle |
T2a Tumor invades superficial muscle (inner half) |
T2b Tumor invades deep muscle (outer half) |
T3 Tumor invades perivesical tissue |
T3a Microscopically |
T3b Macroscopically (extravesical mass) |
T4 Tumor invades any of the following: prostate, uterus, vagina, pelvic wall, abdominal wall |
T4a Tumor invades prostate, uterus or vagina |
T4b Tumor invades pelvic wall or abdominal wall |
N: Lymph nodes |
NX Regional lymph nodes cannot be assessed |
N0 No regional lymph node metastasis |
N1 Metastasis in a single lymph node in the true pelvis (hypogastric, obturator, external iliac or presacral) |
N2 Metastasis in multiple lymph nodes in the true pelvis (hypogastric, obturator, external iliac, or presacral) |
N3 Metastasis in common iliac lymph node(s) |
M: Distant metastasis |
M0 No distant metastasis |
M1 Distant metastasis |
2 General Treatment Concepts for Urothelial Cancers in Radiation Oncology
2.1 Treatment Concepts in Superficial, Non-muscle Invasive Bladder Cancer (Ta, Tis, T1)
Over 80 % of newly diagnosed cases are classified as Ta, Tis or T1. TUR (transurethral resection) is the cornerstone of initial treatment. In case of risk factors, additional intravesical cytostatic therapy, either with cytotoxic drugs (e.g. mitomycin C) or with Bacillus Calmette-Guérin (BCG) is recommended. The efficacy of intravesical therapy with regard to reduction of local recurrence rate and delaying progression is evident, but a significant impact on long-term outcome and survival remains controversial. Cystectomy is considered as standard therapy in case of poor-prognostic superficial cancers with failure after TUR and intravesical therapy (especially in recurrent T1 G3 cancers).
There are few data on the use of radiotherapy. Some older series from the Netherlands have demonstrated good results with TUR and adjuvant interstitial brachytherapy of the tumor area. Data on external beam radiotherapy are very limited. In a prospective series from Erlangen University, Germany, external beam radiotherapy plus concurrent chemotherapy was used for poor prognostic T1 tumors which otherwise would have been considered candidates for cystectomy. The results with regard to overall survival compare favourably to the results of series with cystectomy. The bladder-preservation rate was about 65 % suggesting that an organ-preserving approach is justified even in non-muscle invasive tumors which otherwise would be treated with cystectomy (Weiss et al. 2006).
2.2 Treatment Concepts and Therapy Results in Localised Muscle-Invasive Bladder Cancer (T2-4 N0/1 M0)
Although radical cystectomy has been considered as standard for localised muscle-invasive bladder cancer, there is rapidly growing evidence from numerous phase-II studies that concurrent radiochemotherapy yields survival rates identical to surgical series but with the chance of bladder preservation in 70–80 % of long-term survivors. The most impressive advantage with regard to survival has recently been shown in the British BC 2001-study which demonstrated the superiority of concurrent chemoradiation over radiotherapy alone (James et al. 2012). The absolute difference in survival was 13 % after 5 years which is higher than the increase in survival with adjuvant or neoadjuvant chemotherapy prior or after radical cystectomy. Thus, radiochemotherapy is the standard of care for organ preservation and is, with regard to survival, to be considered as equieffective to cystectomy according to the best available evidence at the moment.
2.3 Indications for Definitive Curative Radiotherapy
Radiotherapy is an attractive curative option for nearly all patients with muscle invasive bladder cancer with the additional advantage of bladder preservation in the majority of patients. Standard treatment for patients with curative approach is radiotherapy plus concurrent chemotherapy. The best chemotherapy regimen is not yet clear. The most widely used drug has been cisplatin (Roedel et al. 2002). However, a combination of 5-FU and mitomycin C has also demonstrated significant efficacy in the BC 2001-trial and is surely an evidence-based alternative to cisplatin (James et al. 2012). A further drug for radiosensitization is paclitaxel which has been shown to be safe and efficacious in patients with contraindications to cisplatin (Mueller AC et al. 1977). There are no obvious contraindications against an organ-preserving approach with combined radiochemotherapy except patients with previous pelvic radiation treatment or other situations that limit the administration of a curative radiation dose.
All patients should initially undergo a complete transurethral resection of the bladder tumor (TUR-BT). The TUR should be complete, if possible; a macroscopically complete TUR-BT is a major prognostic factor. TUR-BT is followed by chemoradiotherapy. About 6 weeks after chemoradiotherapy, a control cystoscopy is recommended. A pathologically complete remission (pCR) can be achieved in about 70 % of all patients and a pCR is a prognostic factor for survival, long-term tumor control in the bladder and bladder preservation (Jenkins et al. 1988; Shipley et al. 1997; Mameghan et al. 1995; Roedel et al. 2002). Patients with residual invasive tumor on control cystoscopy should undergo salvage cystectomy, if there are no contraindications to major surgery.
Start of radiotherapy is recommended 4–6 weeks after TUR-BT because some time is normally required until local symptoms after TUR (dysuria, urgency) have been solved.
2.4 Adjuvant Radiotherapy
The role of adjuvant radiotherapy is not well defined with very limited data. Preoperative radiotherapy has been used in historical studies but is not considered as standard in contemporary guidelines. Preoperative chemoradiation followed by cystectomy is, from a theoretical point of view, an attractive concept, but should only be used in clinical trials.
2.5 Palliative Radiotherapy
Palliative radiotherapy is useful for the relief of symptoms such as bleeding and reduces the pain for patients with T4 bladder tumors, pelvic nodal disease, bone and other distant metastases. Short courses of palliative pelvic radiotherapy may be beneficial for elderly patients who have significant comorbidities precluding radical treatment.
3 Efficacy and Toxicity of Radiotherapy
3.1 Remission Rate and Local Control
In several large prospective series, efficacy of therapy has been determined by cystoscopic evaluation of remission rates. The overall remission rates that have been reported are in the range of 60–70 %. Major prognostic factor that have consistently been reported are the completeness of the TUR-BT prior to radiotherapy and the use of chemotherapy. The completeness of TUR has in some series been classified as visibly complete or incomplete. In one large prospective series, visibly complete TUR was further subdivided in pathologically complete (R0) or incomplete (R1). This subdivision enabled the definition of a very favourable group (T2-3 R0) with a very high rate of clinical remission (90 %) and overall survival (Dunst et al. 2001). Moreover, within the group of patients with histologically proven complete TUR (R0-resection), there was no difference between cT2- and cT3-tumors. Thus, the impact of established prognostic factors depends on the parameters which are included in a multivariate model.
3.2 Overall Survival
The overall survival of patients treated in organ-sparing protocols lies in the range of about 50 % after 5 years which is nearly identical to cystectomy series. One might assume that at least a subset of radiotherapy patients is referred to radiotherapy because of contraindication to major surgery. Being unfit for surgery, however, is a major prognostic factor for survival and identical survival figures after radiotherapy as compared to cystectomy despite a possibly negative selection further support the efficacy of radiotherapy.
Major prognostic factors for overall survival are: age, T-category, completeness of TUR, and remission after radiochemotherapy.
3.3 Bladder Preservation
Bladder preservation can be achieved in about 70–80 % of patients undergoing an organ-preserving approach. These figures have very consistently been reported in the literature. The percentage of bladder-preservation in long-term survivors is in the same range resulting in a bladder-intact survival after 5 years of about 40 % (Roedel et al. 2002; Efstathiou et al. 2012).
Major prognostic factors for bladder-preservation are: completeness of TUR, remission after radiochemotherapy and T-category.
3.4 Acute Toxicity
The acute toxicity of radiotherapy is moderate. Most patients experience mild to moderate symptoms, mainly GU-symptoms such as frequency, urgency, increased nocturia and reduced voiding intervals. Severe acute rectal and bowel symptoms (grade 3 to 4) have been reported each in about 5 % of patients. In patients receiving simultaneous chemotherapy, additional chemotherapy-related symptoms occur frequently. Severe symptoms (grade 3 to 4) have been reported in terms of haematological toxicity (leucopenia, thrombocytopenia, each about 20–30 %), nausea and vomiting (about 5 %) and elevation of serum creatinine (about 5 %). An impact of patient-related parameters or tumor characteristics to the frequency or severity of acute reactions has not been reported. The only risk factor is the administration of chemotherapy.
3.5 Late Toxicity
Late toxicity of organ-preserving treatment protocols is low and compares favourably to series with radical cystectomy. The two largest series in the literature (Rödel et al. 2002; Efstathiou et al. 2009) cover more than 700 patients with follow-up times of more than 5 years and have reported consistent data. Moderate late GU-toxicity (mainly increased voiding frequency and intermittent dysuria) were observed in about 20–25 % of all patients. Grade 3 and 4 late toxicity comprises of GU-toxicity (about 15–20 %) and GI-toxicity (about 5–10 %). Grade 4-complications requiring surgery were observed in less than 5 % of patients. Loss of bladder due to complications (cystectomy due to radiation-related side effects like contracted bladder) was noted in 2.5 % of patients with preserved bladder and local tumor control. In both analyses, no prognostic factors for late toxicity have been reported.
4 Nomograms
4.1 Prognostic Factors and Nomograms in Patients Undergoing Cystectomy
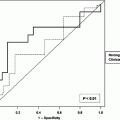
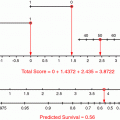
For patients who have undergone radical cystectomy, the most important prognostic factors are pT-category, R0-resection or margin status, pathologically proven lymph node involvement or lymph node density, and (in some other series) elevated CRP and age (Ali-El-Dein et al. 2013; Fairey et al. 2012; Fossa et al. 1996; Xylinas et al. 2012; Yafi et al. 2011). Other factors such as grading or specific histological features seem to be less important. Recently, the impact of molecular features has been stressed. On the basis of these data, a variety of nomograms have been proposed (Table 3). The disadvantage of these factors is that most of them (and especially the most relevant ones) can only be derived from pathohistological examination; this, however, is not possible in patients undergoing a non-surgical approach.
Table 3
Studies with nomograms and prognostic models in patients undergoing cystectomy for urothelial bladder cancer
Author | Data base | Prognostic factors in multivariate model and/or use of nomogram |
|---|---|---|
Fossa et al. (1996) | Single-institution analysis of 534 patients treated with preop. XRT and cystectomy or definitive radiotherapy | T category, trial participation, treatment, creatinine, haemoglobin, age and time since initial diagnosis |
Bochner et al. (2006) | International cohort study, 4462 cystectomy patients, treatment period 1969–2004 | Indication for adjuvant chemotherapy can better be predicted by nomogram than by stage, resulting in less chemotherapy without impairment of survival |