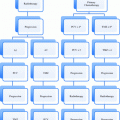
FIGO
TNM
Description
–
TX
Primary tumor cannot be assessed
–
T0
No evidence of primary tumor
–a
Tis
Carcinoma in situ (pre-invasive carcinoma)
I
T1
Cervical carcinoma confined to uterus (extension to corpus should be disregarded)a
IA
T1a
Invasive carcinoma diagnosed only by microscopy (all macroscopically visible lesions are stage IB/T1b tumors). Stromal invasion with a maximum depth of 5.0 mm measured from the base of the epithelium and a horizontal spread of 7.0 mm or less. Vascular space involvement, venous or lymphatic, does not affect classification
IA1
T1a1
Measured stromal invasion 3.0 mm or less in depth and 7.0 mm or less in horizontal spread
IA2
T1a2
Measured stromal invasion more than 3.0 mm and not more than 5.0 mm, with a horizontal spread 7.0 mm or less
IB
T1b
Clinically visible lesion confined to the cervix or microscopic lesion greater than IA1/IA2
IB1
T1b1
Clinically visible lesion 4.0 cm or less in greatest dimension
IB2
T1b2
Clinically visible lesion more than 4.0 cm in greatest dimension
II
T2
Cervical carcinoma invades beyond uterus but not to pelvic wall or to lower third of vagina
IIA
T2a
Tumor without parametrial invasion
IIA1
T2a1
Lesion 4.0 cm or less in greatest dimension
IIA2
T2a2
Lesion more than 4.0 cm in greatest dimension
IIB
T2b
Tumor with parametrial invasion
III
T3
Tumor extends to pelvic wall and/or involves lower third of vagina, and/or causes hydronephrosis or nonfunctioning kidney
IIIA
T3a
Tumor involves lower third of vagina, no extension to pelvic wall
IIIB
T3b
Tumor extends to pelvic wall and/or causes hydronephrosis or nonfunctioning kidney
IV
T4
Bladder and/or rectal invasion or distant spread
IVA
T4a
Tumor invades mucosa of bladder or rectum, and/or extends beyond true pelvis (bullous edema is not sufficient to classify a tumor as IVA)
IVB
T4b
Distant metastasis (including peritoneal spread, involvement of supraclavicular or mediastinal lymph nodes, lung, liver, or bone)
3/4
Nx
Regional lymph nodes cannot be assessed regional lymph node metastasis
3/4
N0
No regional lymph node metastasis
3/4
N1
Regional lymph node metastasis
3/4
M0
No distant metastasis (no pathologic M0; use clinical M to complete stage group)
3/4
M1
Distant metastasis (including peritoneal spread, involvement of supraclavicular or mediastinal lymph nodes, lung, liver, or bone)
Despite the fact that the FIGO staging system suffers from the aforementioned limitations, it remains the current standard of practice, and provides the major entrance criteria utilized in determining the eligibility of patients for cooperative group trials. Thus, most current cooperative group trials enroll patients across almost the entire FIGO stage spectrum, from stage IB2-IVA, and accession them to largely uniform treatment regimens. Of note, although cross-sectional imaging is not “permitted” to influence FIGO stage assignment, the use of CT, MRI and molecular imaging with fluorodeoxyglucose (18F)FDG PET imaging is likely to result in “stage migration” by excluding patients with subtle imaging-based evidence of regional or distant metastatic involvement from cooperative group trials (Fig. 1). This will make any improvements of therapy with newer interventions difficult to compare to historic controls. However, the incorporation of functional imaging into future clinical trials will potentially enhance our ability to accurately stratify patients and tailor therapy to the “true” clinical stage (i.e. locally advanced vs. metastatic disease).
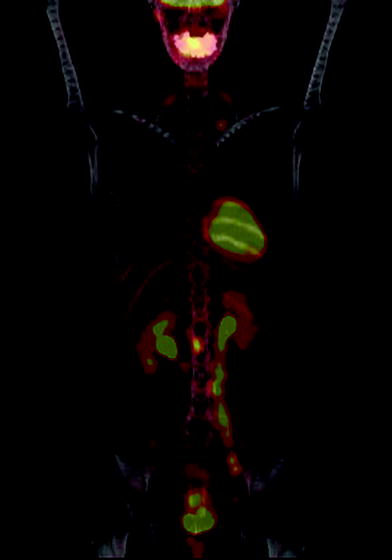

Fig. 1
PET-CT and Staging of Cervical Cancer: A 43 year old woman with invasive squamous cell carcinoma of the cervix underwent PET-CT revealing retroperitoneal and supraclavicular adenopathy, consistent with Stage IV disease
2.2 Clinical Factors
Eligibility for primary surgical therapy is determined by regional tumor extent to adjacent structures and significantly influences prognosis. Patients with stage I disease (tumor limited to the cervix) and selected patients with stage II disease (including patients with upper vaginal involvement), are candidates for radical hysterectomy. The overall survival of surgically treated patients with stage IB tumors ranges from 85 to 90 % (Morley and Seski 1976; Hopkins and Morley 1991; Landoni et al. 1997). However, large tumor size, deep cervical invasion, lymphovascular space invasion, as well as involved lymph nodes and parametrial involvement have been recognized as risk factors for pelvic recurrence after radical hysterectomy. Depending on the number and extent of these factors present, adjuvant therapy can improve outcomes, albeit at the cost of increased risk of toxicity from adjuvant radiation and/or chemotherapy (Sedlis et al. 1999; Peters et al. 2000; Rotman et al. 2006). Thus, if imaging modalities or other factors could identify the presence of these pathologic features during workup leading to upstaging, definitive radiation and chemotherapy could be considered instead of primary surgery, thus potentially reducing the morbidity of treatment.
2.2.1 Stage
In patients with cervical cancer treated with definitive radiation therapy, FIGO stage remains an important prognostic factor. Due to the relative rarity of cervical cancer in western nations, phase III cooperative group trials do not subclassify patients by stage, nor do they group patient cohorts as stages IB–II versus III–IVA for subgroup analyses, because analysis by individual stage categories would require unachievably large patient cohorts. Based on large single-institution series in which contemporary radiation techniques and concurrent chemotherapy were utilized, reported local control rates, disease free survival rates, and overall survival rates for patients with Stage IB–IIA and III–IVA are 87 and 79 %, 74 and 54 %, and 79 and 59 %, respectively (Whitney et al. 1999; Eifel et al. 2004; Rose et al. 2007).
2.2.2 Tumor Volume
In addition to FIGO stage, tumor size has profound prognostic significance (Eifel et al. 1994; Kovalic et al. 1991). In 1988 FIGO added tumor diameter as a stratifying factor for stage I disease, with tumors less than or greater than 4 cm classified as stage IB1 versus IB2 respectively. In the 2009 revision of the staging system, tumor size of greater or less than 4 cm was also incorporated into the stage IIB category (Pecorelli et al. 2009). Tables 2 and 3 shows the profound significance of tumor size, measured as largest or average palpated diameter, for local control and survival. Within the same stage category of IB, tumor size of <4 cm in diameter was associated with a disease-free survival of 87 %, compared to 72 % for 6 cm, 69 % for 7 cm, 64 % for 8 cm and 47 % for >8 cm tumors (Table 2). Similar relationship exists for tumor size and outcomes within the stage IIB category (Table 3) (Eifel et al. 1994; Hansgen and Dunst 1996; Hockel et al. 1996; Homesley et al. 1980; Lowrey et al. 1992; Perez et al. 1992b; Mendenhall et al. 1984).
Table 2
Tumor diameter versus disease free survival for stage IB cervical cancer
Size (cm) | NP | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | >8 |
|---|---|---|---|---|---|---|---|---|---|---|
Eifel et al. (1994) n = 1,526 | 94 | 87 | 86 | 72 | 69 | 64 | 47 | |||
Lowrey et al. (1992) n = 130 | 93 | 77 | 67 | |||||||
Perez et al. (1992b) n = 384 | 90 | 65 | ~60 | |||||||
Homesley et al. (1980) n = 45 | 95 | 67 | ||||||||
2.2.3 Lymph Node Status
For any given FIGO stage, lymph node involvement reduces overall survival by approximately 50 % (Stehman et al. 1991). Furthermore, among patients with positive lymph nodes, prognosis declines with increasing extent of lymph node involvement (Macdonald et al. 2009; Hsu et al. 1972; Tsai et al. 1999; Takeda et al. 2002; Morice et al. 1999). In a pooled study by the Gynecologic Oncology Group (GOG), para-aortic involvement was associated with an 11-fold risk of recurrence and sixfold risk of death, and was also associated with extrapelvic failures (Berman et al. 1984). However, even with para-aortic lymph node involvement, survival in the range of 20–50 % has been reported for patients with locally advanced disease (Komaki et al. 1983; Rotman et al. 1994), justifying aggressive therapy for patients with regional lymphatic spread.
Although controversy exists whether surgical excision of suspicious lymph nodes improves outcomes, a large retrospective study of patients treated in the pre-chemo-radiation era showed among patients who underwent lymphadenectomy and postoperative radiation, patients with macroscopically involved lymph nodes had similar regional and distant tumor control as those with microscopic lymph node involvement, and significantly better than those patients with unresectable lymph nodes (Cosin et al. 1998). This supports the use of imaging for identification of involved nodes, thus allowing for a tumor directed combined modality approach. Increasing use of molecular imaging in cervix cancer will facilitate this approach and will also likely lead to stage migration as lymph nodes with more subtle involvement can be identified and treated more aggressively.
2.3 Patient Factors
2.3.1 Hemoglobin
Over the past 50 years, numerous studies have provided indirect evidence that the effects of poor tumor blood supply have an adverse impact on radiation response. Early studies of morphologic parameters of angiogenesis, such as microvessel density, have been shown to correlate with radio-responsiveness and clinical outcome in cervical cancer (Awwad et al. 1986; Cooper et al. 1998). Cervical cancer patients with high inter-capillary distances locally within their tumors measured by colposcopy were found to have increased tumor recurrence rates after radiation therapy (Kolstad 1968).
Similarly cervical cancer patients with low hemoglobin levels have been reported to have higher recurrence rates after radiotherapy (Mendenhall et al. 1984; Bush et al. 1978; Evans and Bergsjo 1965; Diesche et al. 1983; Thomas 2001; Dunst et al. 2003). This supports the concept that poor “systemic” oxygenation is clinically significant for treatment outcome. Haensgen et al. analyzed hemoglobin levels of 70 patients, and reported survival was 27 % for patients with low hemoglobin (<11 g/dL), compared to 62 % in those with higher levels (Haensgen et al. 2001). Dunst et al. mirrored these results, showing overall survival of 64 and 32 %, respectively and local recurrence rates of 15 % versus 67 %, respectively (Dunst et al. 2003). Hemoglobin during the course of therapy, when the actual cytotoxic events occur, may also be relevant. Thomas et al. showed in 605 patients that the average weekly hemoglobin nadir <12 g/dL was associated with a higher incidence of local failure and metastases (Thomas 2001). In a recent study, weekly mean hemoglobin levels measured during the course of radiotherapy was more predictive of outcome than pre-therapy or nadir hemoglobin (Mayr et al. 2009). In all, the thresholds value for this effect of hemoglobin level appears to be in the range of 11–12 g/dL.
Although the impact of blood transfusion on outcome in patients treated with definitive radiation therapy remains controversial, the Canadian experience suggests that maintaining hemoglobin levels above 12 g/dL is associated with improved 5-year survival. Pre-treatment hemoglobin, which may not reflect the longitudinal status of hemoglobin levels, did not have any impact on outcome (Grogan et al. 1999). Interestingly, in one retrospective study of 204 patients at a single institution where departmental practice was to transfuse for hemoglobin <11 g/dL, it was noted that only 18.5 % of patients who received transfusion had a sustained response to transfusion, although outcomes for these patients were equivalent to those presenting with normal hemoglobin (Kapp et al. 2002). However, for patients who did not have a sustained response to blood transfusion, outcomes were significantly worse compared to those with response or with normal hemoglobin pre-therapy. While there was a therapeutic benefit to transfusion for those patients with sustained response, the low rate of response of 18.5 % was disappointing, and it was proposed that finding and treating the underlying cause of the anemia may be more beneficial.
2.4 Histologic Factors
2.4.1 Histology
Approximately 90 % of cervical cancers are squamous cell carcinomas. Squamous cell carcinomas arise from epithelial precursors, and can be classified into one of three cell types: large cell keratinizing, large cell nonkeratinizing, and small cell. Tumor grade is based on the degree of differentiation, and is reported as well, moderately, or poorly differentiated. Adenocarcinoma is the second most common, accounting for 10–15 % depending on region and age. More recently, the incidence of adenocarcinomas appears to be increasing, especially in younger patients (Liu et al. 2001; Smith et al. 2000). Adenocarcinomas arise from the mucus-secreting endocervical glands of the cervix or the cylindrical mucosa. The most common subtype of adenocarcinoma of the cervix is endometrioid adenocarcinoma, where cells have characteristic features of the endometrium and grading is based on the degree of gland formation. It is critical to differentiate this from primary endometrioid endometrial adenocarcinoma as recommended therapy would change, thus clinical presentation, such as absence or presence of an endometrial tumor with extension into the cervix, is incorporated to determine the true site of primary disease. The next most common subtype of adenocarcinoma is adenosquamous histology, comprising 21–30 % of adenocarcinomas (Farley et al. 2003; Kleine et al. 1989), and is characterized by epithelial cell cores mixed with glandular structures. Other histologies, such as clear cell, small cell carcinoma, basaloid carcinoma, lymphoma, and sarcomas occur, but are rare and have varying prognostic impact.
The prognosis of adenocarcinoma versus squamous cell histology is debated. While adenocarcinoma is associated with an increased risk of failure, particularly metastatic failure in some retrospective reports (Eifel et al. 1995; Huang et al. 2011, 2012), many show no significant impact on outcome between adenocarcinoma and squamous cell carcinoma (Shingleton et al. 1995; Look et al. 1996; Davidson et al. 1989). Interestingly, adenosquamous carcinoma may be associated with poorer recurrence free and overall survival (Farley et al. 2003; Look et al. 1996; Lea et al. 2003; Grisaru et al. 2001; Galic et al. 2012). In all, the differences in outcomes among these studies may be in part due to regional variation in Human Papilloma Virus (HPV) genotype distribution, changes in etiology and incidence of histologic type, differences in treatment approach, and overall study sizes, making it difficult to draw any definitive conclusion about subtype implications in the absence of prospective data.
2.4.2 Histopathologic Risk Factors in Postoperative Patients
In surgically treated stage I-IIA patients, lymph node involvement, parametrial invasion and involved margins have long been recognized as high risk factors for local recurrence and death (Morrow 1980). In those with involved lymph nodes, number of involved nodes (<3 vs. >3), bilaterality, level (common iliac vs. pelvic) and size (micro- vs. macroscopic) impact outcome (van Bommel et al. 1987; Tanaka et al. 1984). Therefore, adjuvant therapy based on histopathologic risk factors is paramount because salvage therapy for recurrent cervical cancer after hysterectomy has dismal results with a 5–45 % survival (Thomas et al. 1993). Postoperative radiation has been the hallmark in adjuvant therapy.
Tumor size, depth of invasion and capillary-lymphatic space invasion have also been shown to impact prognosis in surgically treated stage I-IIA patients. However, until the completion of the phase III GOG 92 study, the impact of adjuvant therapy on survival was not well established. GOG 92 (Sedlis et al. 1999; Rotman et al. 2006) established a set of intermediate risk factors (commonly referred to as “Sedlis criteria”) for poor outcome in stage IB patients treated with radical hysterectomy. Patients with two of the three features (capillary lymphatic space invasion, large clinical tumor diameter, or more than one-third cervical stromal invasion) were randomized to pelvic radiotherapy versus no further therapy (Table 4). At 10 years median follow-up, postoperative radiation reduced the risk of recurrence by 46 % (HR 0.54) with the greatest benefit in patients with a combination of deep 1/3 invasion plus tumor size >4 cm (HR 0.16) or capillary lymphatic space invasion plus deep 1/3 invasion with any tumor size (HR 0.53) (Rotman et al. 2006). There was no significant improvement in overall survival (Table 5). On subgroup analysis, proportionally greater improvement was noted among 44 patients with adenosquamous or adenocarcinoma, where adjuvant radiation therapy reduced the recurrence rate from 44 to 9 % (Rotman et al. 2006). A current GOG study is underway to evaluate whether postoperative radiation with concurrent chemotherapy can further improve upon this outcome.
Table 4
Inclusion criteria for GOG 92: randomization to postoperative pelvic radiotherapy versus no further therapy in stage IB intermediate risk cervical cancer
LVSI | Depth of invasion | Tumor size (cm) |
|---|---|---|
Positive | Deep 1/3 | Any |
Positive | Middle 1/3 | ≥2 |
Positive | Superficial 1/3 | ≥5 |
Negative | Deep or middle 1/3 | ≥4 |
Table 5
GOG 92 results: postoperative radiotherapy improves recurrence-free, but not overall survival, in intermediate risk stage IB cervix cancer
RT (n = 137) (%) | Observation (n = 140) (%) | p value | |
|---|---|---|---|
Recurrences (all) | 17.5 | 30.7 | 0.007 |
AC, AS | 8.8 | 44.0 | 0.019a |
Squamous cell | 20.4 | 27.8 | |
Survival | 80.3 | 71.4 | n.s. |
Improvement of adjuvant therapy with the addition of concurrent chemotherapy to radiation has also been demonstrated for some select patients. The intergroup trial GOG 109 randomized stage IA2-IIA patients treated with radical hysterectomy and pelvic lymphadenectomy and high risk features, defined as positive pelvic lymph nodes and/or positive margins, and/or microscopic involvement of the parametrium, to pelvic radiotherapy versus pelvic radiotherapy with chemotherapy (cisplatin/5-FU for 4 cycles during and after radiation) (Peters et al. 2000). Addition of chemotherapy resulted in significant improvement of overall survival at 81 % versus 71 % (HR 1.96, p = 0.007). The greatest benefit was observed for patients with larger tumors and multiple involved lymph nodes, underscoring the importance of identification of involved lymph nodes in order to offer the optimal adjuvant therapy. Table 6 summarizes the results of five randomized trials that show improved survival with concurrent chemotherapy and radiotherapy (Peters et al. 2000; Whitney et al. 1999; Rose et al. 2007; Keys et al. 1999; Morris et al. 1999).
Table 6
Estimates of the relative risk of death in five clinical trials of radiotherapy and concurrent chemotherapy
Study | FIGO stage | Control group | Comparison group | Relative risk of death | p value |
|---|---|---|---|---|---|
Peters et al. (2000) | IB or IIA | RT | RT plus cisplatin and 5-FU | 0.5 | 0.007 |
Whitney et al. (1999) | IIB–IVA | RT plus hydroxyurea | RT plus cisplatin and 5-FU | 0.72 | 0.018 |
Rose et al. (2007) | IIB–IVA | RT plus hydroxyurea | RT plus weekly cisplatin RT plus cisplatin, 5-FU, hydroxyurea | 0.61 0.58 | <0.025 <0.025 |
Keys et al. (1999) | IB2 | RT | RT plus weekly cisplatin | 0.54 | 0.008 |
Morris et al. (1999) | IB–IVA | Extended field RT | RT plus cisplatin and 5-FU | 0.52 | 0.004 |
2.4.3 Molecular Tumor Markers
2.4.3.1 HPV
HPV is found in an estimated 93–99.7 % of invasive cervical cancer (Bosch et al. 1995; Walboomers et al. 1999). Further, the prevalence of different genotypes varies in cellular histology. HPV16 is identified in the majority of squamous cell carcinomas, and HPV18 is the predominant genotype in adenocarcinomas and adenosquamous carcinomas (Bosch et al. 1995). HPV may be a prognostic indicator for outcomes. Several studies have shown HPV18 and HPV16 is associated with more advanced cervical cancers at presentation and poorer outcomes (Schwartz et al. 2001; Pilch et al. 2001; Burger et al. 1996). Further, HPV18 has been associated with increased radioresistance and increased recurrence rates compared to other HPV genotypes in patients receiving only radiation therapy (Wang et al. 2010). However, HPV18 has subsequently been shown to be predictive of improved disease specific survival when concurrent chemotherapy and radiotherapy was used in place of radiotherapy alone (Wang et al. 2012). The clinical utility of this association is an area of active investigation.
2.4.3.2 Angiogenesis
Angiogenesis-related molecular markers would be expected to be of great importance for radiation and chemotherapy because of the critical dependence of the cytotoxic effect on tumor microcirculation and oxygenation (Tannock 1972). It is postulated that poorly-perfused, hypoxic, endophytic tumors are associated with radio-resistance and resulting poor treatment outcome in cervical cancer. Angiogenic factors have been shown to correlate with tumor recurrence and survival in surgically treated patients (Cheng et al. 2000; Dellas et al. 1997; Dinh et al. 1996; Hawighorst et al. 1997; Lee et al. 2011; Mayr et al. 1999; Kainz et al. 1995; Obermair et al. 1998; Tjalma et al. 2000). Therefore, there has been increasing interest in molecular markers of angiogenesis and cytokines in cervical cancer. Cooper et al. (Cooper et al. 1998) reported that patients with high MVD had significantly poorer local control and survival. Although Gaffney et al. (2003) found increased VEGF and EGFR expression to be associated with poor survival, inconsistent results have been observed in regard to VEGF association with tumor progression, stage (Loncaster et al. 2000), histologic type (Cheng et al. 2000; Loncaster et al. 2000, 2002) and microvessel density (MVD) (Mayr et al. 1999; Hawighorst et al. 1998). High expression of another angiogenic marker, carbonic anhydrase IX (CA IX) correlates with poor survival (Loncaster et al. 2002). More recently the GOG evaluated a panel of angiogenesis markers including MVD, VEGF, CD31 (non-specific endothelial marker), TSP-1 (thrombospondin-1 an anti-angiogenesis factor), and CD105 (tumor-specific endothelial marker) and association with clinical outcome (Randall et al. 2009). Expression of each was determined in tumors from patients included in GOG 109, including stage IA2-IIA patients with positive lymph nodes, parametrial involvement, or positive surgical margins (Peters et al. 2000). Of these, only high expression CD31 was independently predictive of improved disease free and overall survival. Authors posit that this may be representative of CD31 as a surrogate marker for improved tumor flow and oxygenation, thus improving response to adjuvant therapy.
2.4.3.3 Alternate Candidate Molecules
There has been increasing interest in evaluation of molecular mechanisms of radiation response through candidate gene approach and microarray analysis. Studies in cervical cancer cell lines have found that genes related to angiogenesis, apoptosis and tumor cell invasion correlate with radio-resistance (Harima et al. 2004; Kitahara et al. 2002; Tewari et al. 2005; Wong et al. 2003). A pilot study of 12 patients in 2008 used microarray analysis and demonstrated immortalization upregulated protein (IMUP), IGF-2, and ARHD were associated with tumor recurrence in patients treated with radiation and concurrent chemotherapy (Klopp et al. 2008). Proteins that have been shown to correlate with clinical outcome include Ku80, GADD45 (Harima et al. 2003), bax, bcl-2 (Harima et al. 1998), intracellular adhesion molecule-3 (ICAM-3) (Chung et al. 2005), and hypoxia inducible factor (HIF)-1a (Bachtiary et al. 2003; Burri et al. 2003). However, to date, none of these molecular markers has been incorporated into clinical care.
2.5 Imaging Prognostic/Predictive Markers
2.5.1 Morphologic Imaging
Improvements in spatial and temporal resolution of cross-sectional imaging have broadened the capabilities of both anatomical and functional imaging in cervical cancer. Three-dimensional tumor volume can be quantified, and tumor extent and involvement of adjacent structures more accurately assessed than by clinical palpation (Hricak et al. 1988; Hricak 1991; Bhosale et al. 2010; Balleyguier et al. 2011). Higher temporal and spatial resolution also allows for functional imaging, such as dynamic contrast-enhanced (DCE) MRI and diffusion-weighted imaging, in addition to the morphologic/anatomical imaging. Beyond pre-therapy assessment, repeated imaging throughout the course of definitive chemoradiotherapy with an intact cervix provides longitudinal information on functional changes in response to ongoing therapy. Such on-therapy imaging shows promise for deriving imaging biomarkers to predict therapeutic response and disease outcome.
Tumor size in cervical cancer is best assessed with MRI (Bhosale et al. 2010; Balleyguier et al. 2011), which was demonstrated in imaging-histologic correlation studies (Burghardt et al. 1989; Greco et al. 1989). For on-therapy assessments, the velocity of tumor regression, assessed by 3D tumor volumetry (not diameter-based measurement) allows an indirect measure of therapy responsiveness (Mayr et al. 2006), which has been shown to be predictive of treatment outcome in cervical cancer patients treated with radiation/chemotherapy (Hatano et al. 1999; Mayr et al. 1996, 2010; Sethi et al. 2005; Lim et al. 2008). Using 3D volumetric measurements, Mayr et al. found that patients with <20 % of residual tumor volume at 40–50 Gy delivered over 4–5 weeks had excellent local control and disease free survival of 90.5 and 88.4 %, compared to 23.1 and 45.4 % in patients with slower tumor regression (Mayr et al. 1996). Similarly, Hatano et al. (1999) found 100 % local control in patients with rapid tumor volume regression to less than 30 % of the original volume at 30 Gy over 3 weeks. Further, the velocity of tumor shrinkage directly correlates with patients’ risk for local failure and death of disease (Mayr et al. 2010). Such early predictive information, available during the ongoing therapy course, may open a window of opportunity to adapt and intensify therapy. For post-therapy assessment in the early follow-up period, complete resolution of the tumor 3–6 months after therapy is associated with better outcome (Hricak 1991; Flueckiger et al. 1992).
2.5.2 Functional Imaging
Among the functional imaging modalities, DCE MRI provides an in vivo imaging biomarker that indirectly reflects tumor perfusion and the delivery of oxygen and therapeutic agents to the tumor. Low perfusion, indicative of poor vascularity and oxygenation, before or early during the course of radiation therapy (at approximately 20 Gy, ~2 weeks), significantly predicts unfavorable local tumor control (73 % vs. 100 %, p = 0.006) and survival (47 % vs. 79 %, p = 0.001, respectively). The 2-week intra-treatment time point may be superior to the pre-therapy time point likely because the 2-week DCE MRI incorporates early therapy-specific information of responsiveness to the ongoing treatment (Yuh et al. 2009).
Diffusion-weighed imaging, which indirectly assesses tumor cellularity (Hamstra et al. 2008; Ross et al. 2003) provides another imaging biomarker in cervix cancer. The apparent diffusion coefficient (ADC) measures the magnitude of diffusion (of water molecules) within tissues. A low ADC value is indicative of increased tissue cellularity, and an increase in the ADC suggests cell death. Such an ADC increase can occur very early, within days of therapy start, prior to any morphologic changes (e.g. tumor volume) (Charles-Edwards and DeSouza 2006; Charles-Edwards et al. 2008; Chenevert et al. 2000). Early clinical experience shows that increase in ADC during ongoing radiation and chemotherapy correlates with improved tumor response (Harry et al. 2008; Naganawa et al. 2005; Liu et al. 2009). These studies suggest that both DCE-MRI and DW-MRI may have value as early imaging biomarkers of radioresponsiveness in cervical cancer.
In addition to being the most accurate assessment of lymph node involvement, FDG-PET/CT has also been used to assess the primary tumor during/after therapy. Persistent metabolic activity of the tumor 3 months after therapy has been correlated with poor outcome (Kidd et al. 2007). However, the optimal imaging timing for FDG-PET is a subject of active investigation.
3 Cancer of the Uterine Corpus
Endometrial cancer is the most common gynecologic malignancy in the United States. In 2013, 49,500 cases of endometrial cancer are expected, accounting for approximately 6 % of female malignancies, with approximately 8,200 deaths anticipated, accounting for 3 % of all female cancer deaths (Siegel et al. 2013). Mean age at diagnosis in the United States is approximately 62 years old, consistent with a disease largely occurring in postmenopausal women. SEER data show approximately 70 % of cases are diagnosed as localized disease, with an 81.5 % 5-year survival for all stages, and 95.3 % for localized disease (Howlader et al. 2013). Risk factors for endometrial cancer include diabetes, obesity, hyperestrogenic state, nulliparity, tamoxifen use, early menarche or late menopause, and anovulatory cycles (Brinton et al. 1992). Certain genetic diseases, such as hereditary nonpolyposis colon cancer (HNPCC) or Lynch syndrome and Cowden disease, are associated with increased risk for endometrial cancer, with lifetime risks ranging from 10 to 60 % depending on disease and specific genetic mutation (Aarnio et al. 1995; Gustafson et al. 2007).
Most endometrial cancers are diagnosed during the workup of abnormal, or postmenopausal, vaginal bleeding. Pathologic diagnosis is essential, as both FIGO stage and FIGO histologic grade are prognostic for outcome and determine treatment. Thus, diagnosis is often made via endometrial biopsy or dilation and curettage for those patients in which endometrial biopsy is not possible or non-diagnostic. Endometrial cancers often arise within the endometrial layer, and spread by invasion into the myometrium. In more advanced disease, tumor can spread to the uterine serosa, adnexa, endocervical canal, peritoneal cavity, bowel, bladder, and other adjacent structures. Lymphatic drainage is to the pelvic lymph nodes (including the internal/external iliacs, common iliacs, obturator, presacral and parametrial), with direct spread to the para-aortic lymph nodes possible. FIGO staging requires surgical staging based on the at-risk areas of spread, therefore total hysterectomy and bilateral salpingo-oopherectomy, with or without lymph node dissection, is performed in most patients. Adjuvant therapy is then based on pathologic information that determines the stage and grade of each endometrial cancer, both of which are prognostic for patient outcome.
3.1 Staging
The gold standard for staging in endometrial cancer remains surgical staging as defined by FIGO (Creasman 2009), Table 7. Prior to the 1988 FIGO staging system, staging was clinical evaluation for tumor size, extent of disease (confined to uterus or pelvic extension), and bowel or bladder involvement. However, this was found to understage patients approximately 23 % of the time (Creasman et al. 1987). Therefore, FIGO staging was changed to incorporate surgical evaluation and subsequent pathologic information for staging which improved the prognostic accuracy of staging. Initially, myometrial invasion, cervical invasion (including endocervical glandular involvement), adnexal involvement, serosal involvement, positive peritoneal cytology, and lymph node status were factored into staging. On the last revision of the FIGO surgical staging for endometrial cancer (2009), peritoneal cytology and isolated endocervical glandular involvement have been removed from the criteria. Further, myometrial invasion, previously stratified into three levels of involvement, is now subdivided into only two categories; invasion of less than one-half or invasion of one-half or more of the myometrium. Lymph node positive disease is substratified to pelvic lymph node only, or para-aortic lymph node disease (IIIC1 vs. IIIC2). In summary, under 2009 FIGO staging, stage I disease now includes endometrial/myometrial only disease; stage II disease invades cervical stroma; stage III disease is a heterogenous group with IIIA including uterine serosa or adnexal involvement, IIIB involving the vagina, and IIIC1 versus IIIC2 denoting pelvic lymph node only versus any para-aortic lymph node positive disease; stage IV represents metastatic disease to other sites not included above.
Table 7
FIGO and American Joint Committee on Cancer (AJCC 7th edition) TNM staging for endometrial cancer
FIGO staging (2008) | AJCC 7th edn (2009) TNM staginga | Description | ||
|---|---|---|---|---|
Group | T | N | M | |
IA | T1a | 0 | 0 | Limited to the endometrium or invades less than half of the myometrium |
IB | T1b | 0 | 0 | Invades half or more of the myometrium |
II | T2 | 0 | 0 | Invades cervical stromal tissue but does not extend beyond the uterus |
IIIA | T3a | 0 | 0 | Involves serosa and/or adnexa |
IIIB | T3b | 0 | 0 | Vaginal involvement or parametrial involvement |
IIIC1 | T1–3 | 1 | 0 | Metastasis to pelvic lymph nodes |
IIIC2 | T1–3 | 2 | 0 | Metastasis to para-aortic lymph nodes |
IVA | T4 | Any | 0 | Invades bladder mucosa and/or bowel mucosa |
IVB | Any | Any | 1 | Distant metastasis |
Surgical staging at minimum is to include total hysterectomy and bilateral salpingo-oopherectomy (BSO). The role of extended surgical staging, with sampling and/or dissection of the pelvic and para-aortic lymph nodes, is still debated. Given the significant prognostic importance of lymph node metastasis, many advocate for lymph node histologic evaluation, and some have suggested a possible therapeutic benefit to lymphadenectomy, although not been proven in a prospective manner. While older techniques for extended surgical staging required laparotomy, more modern techniques with laparoscopic assisted methods have yielded equivalent nodal yields with reduced morbidity for many experienced gynecologic oncologists (Eltabbakh 2002; Scribner et al. 2002). Given the fact that many women with endometrial cancer are elderly, obese, and have co-morbidities such as diabetes, hypertension, and coronary artery disease, concerns exist for increased risks of deep venous thrombosis, vascular injury, or pulmonary emboli in the postoperative setting. Further, extended surgical staging followed by adjuvant radiation therapy is reported by some to carry higher enteric morbidity than hysterectomy and radiation alone (Lewandowski et al. 1990). Thus, some point to the experience of PORTEC and ASTEC trials as data to support omission of routine lymphadenectomy in low and intermediate risk patients without clinical/palpable adenopathy. PORTEC-1 included intermediate risk stage I patients, all undergoing total hysterectomy and BSO without lymphadenectomy randomized to adjuvant radiotherapy versus observation with 80–85 % overall survival at 5 years (Creutzberg et al. 2000). In ASTEC, intermediate risk patients underwent total hysterectomy-BSO, pelvic washings, and para-aortic lymph node palpation and were randomized to lymphadenectomy or no further surgery, with no statistically significant difference on overall survival at 3 years (ASTEC study group et al. 2009). Conversely, several studies support the role of maximal surgical debulking and resection of gross nodal disease, with improvement in median survival in some cohorts from 8.8 to 37.5 months (Bristow et al. 2003; Chi et al. 1997; Lambrou et al. 2004).
Of note, the American College of Obstetricians Gynecologists (ACOG) recommends comprehensive surgical staging including total hysterectomy and BSO, pelvic washings, bilateral pelvic and paraaortic lymphadenectomy, and complete resection of all disease, with exceptions considered for young or perimenopausal women with grade 1 endometrioid adenocarcinoma associated with atypical endometrial hyperplasia and those at increased risk of morbidity/mortality secondary to comorbidities (American College of Obstetricians and Gynecologists 2005). Omental sampling is also often performed, especially in papillary serous and clear cell histology due to the risk of upper abdominal spread.
3.2 Clinical Factors
3.2.1 Stage
Surgical stage continues to be one the most important clinical factors predictive of outcomes. The outcomes of 81,900 patients with endometrial cancer from 1988 to 2006 in a SEER database and a cohort of 1,268 patients from the MoMaTEC study were shown to verify the improved prognostic utility of the current 2009 FIGO staging in comparison to the FIGO 1988 staging schema (Lewin et al. 2010; Werner et al. 2012). Five year overall survival rates in early stage disease were 90–96 %, 78–87 %, and 74–80 %, respectively, for stage IA and IB and stage II. In locally advanced disease, 5-year overall survival was 48–56 %, 36–53 %, 57–60 %, and 49–53 % for stage IIIA (serosa/adnexa), IIIB (vaginal), IIIC1 (pelvic lymph node), and IIIC2 (para-aortic lymph node), respectively. Survival in stage IV disease ranged from 16 to 57 %.
3.2.2 Lymph Node Status
Lymph node status is incorporated in the staging classification above. A drop in 5-year overall survival from 74 to 96 % for stage I/II patients to 49–60 % for node positive patients is observed (Lewin et al. 2010; Werner et al. 2012). A variety of features are associated with increased risk for lymph node metastasis. The strong association of tumor grade, depth of myometrial invasion and pelvic lymph node involvement was first demonstrated in the results of GOG study 33 (Tables 8, 9) (Creasman et al. 1987). In this clinical-pathologic study, 621 stage I endometrial cancer patients, accrued from 1977 to 1983, prospectively underwent hysterectomy, selective pelvic and para-aortic lymph node dissection and peritoneal cytology. Increasing FIGO grade and increasing depth of invasion correlated with progressively higher probability of pelvic lymph node involvement, ranging from less than 5 % in patients without myometrial invasion, to 34 % in those with both outer third myometrial invasion and FIGO grade 3 histology.
Table 8
GOG 33: Rate of pelvic lymph node metastasis based on extent of myometrial invasion and FIGO grade
Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | |
|---|---|---|---|
Endometrium only | 0 | 3 | 0 |
Inner 1/3 myometrial invasion | 3 | 5 | 9 |
Middle 1/3 myometrial invasion | 0 | 9 | 4 |
Deep 1/3 myometrial invasion | 11 | 19 | 34 |
Table 9
GOG 33: rate of para-aortic lymph node metastasis based on extent of myometrial invasion and FIGO grade
Grade 1 (%) | Grade 2 (%) | Grade 3 (%) | |
|---|---|---|---|
Endometrium only | 0 | 3 | 0 |
Inner 1/3 myometrial invasion | 1 | 4 | 4 |
Middle 1/3 myometrial invasion | 5 | 0 | 0 |
Deep 1/3 myometrial invasion | 6 | 14 | 23 |
While node positive patients as a whole have poorer survival compared to stage I and II patients, it should be noted that the predictive outcome of node positive disease should be considered in the context of the extent of other extrauterine disease. Mariani et al. examined the outcomes of 51 patients with surgically staged IIIC disease. In this cohort, it was noted that the 5-year recurrence free survival (RFS) for node positive only disease was 68 %, but dropped to 25 % in patients with node positive disease in combination with other extrauterine disease such as adnexal, vaginal, serosal involvement or positive peritoneal cytology (Mariani et al. 2002a). While this study is limited in its correlation to today’s practice as few patient received chemotherapy, this poorer outcome in “higher burden” disease suggests these patient may require a more aggressive treatment approach. The overall nodal disease burden, as described by absolute number of positive lymph nodes and ratio of positive nodes to total nodes on lympadenectomy has also been shown to be prognostic in some studies (Chan et al. 2007a). Five-year disease-specific survival for those with 1, 2–5, and >5 positive nodes were 68.1, 55.1, and 46.1 %, respectively (p < 0.001). Percentage of positive lymph nodes was also evaluated, with 5-year disease-specific survival of 77.3 to 60.7 to 40.9 % in those with ≤0, >10 to ≥50 %, and >50 % nodes involved, respectively. Both factors were independently prognostic on multivariate analysis.
3.2.3 Adnexal and Serosal Involvement
FIGO stage IIIA is defined by serosal and/or adnexal disease spread. Adnexal involvement is associated with poorer outcomes, but is highly correlated with other adverse features such as high tumor grade, other metastatic sites, and unfavorable histology. When considering adnexal involvement in the absence of other factors, outcomes are more favorable than for all stage IIIA patients taken as a whole, with 5-year disease-free survival ranging from 71 to 86 % (Connell et al. 1999; Greven et al. 1989). Serosal involvement is associated with high risk of distant failure, owing in part to its association with other risk factors such as other sites of metastatic disease and higher stage presentation (Greven et al. 1989; Ashman et al. 2001). Similar to adnexal involvement, however, isolated serosal involvement portends an improved prognosis over all patients with serosal involvement, with 5-year disease-free survival of 41.5 % versus 20 % (Ashman et al. 2001).
3.3 Patient Factors
3.3.1 Age
Age has long been considered a risk factor for development of endometrial cancer, as well as prognostic of outcomes. In general, endometrial cancer is a disease of postmenopausal women. Younger women who develop endometrial cancer tend to have improved survival, often with risk factors such as estrogen or other hormone related-disorders, including but not limited to, infertility, polycystic ovarian syndrome, ovarian dysfunction, anovulatory cycles, and obesity (Ota et al. 2005). Young patients tend to have low grade endometrioid histology, correlating to more favorable outcomes.
While many studies have shown advanced age to be an independent predictor of worse outcomes (Kosary 1994; Abeler and Kjorstad 1991; Irwin et al. 1998), many small studies have found this to not be a prognostic factor. Some of been concerned that patient comorbidities, potential de-escalation of therapy in the elderly, or narrow cohorts, or propensity for more advanced stage at diagnosis, or more aggressive histology at diagnosis, among a multitude of other confounding factors, may explain the apparent discrepancy. Regardless, age is still part of the risk stratification of patients for selection of adjuvant therapy as is discussed below.
3.3.2 Serum CA-125
CA-125 is a serum tumor marker that can readily be tested, commonly used to monitor ovarian cancer. The role of CA-125 in endometrial cancer has been proposed to be prognostic, with elevated preoperative CA-125 levels associated with increased risk of lymph node metastasis (Chung et al. 2006). Many suggest measurement of preoperative serum CA-125 given several studies suggestive of prognostic utility (Powell et al. 2005); although no change in therapy is offered based on this value. Some have also proposed an age stratified CA-125 cutoff to improve the predictive value of CA-125 levels, with higher cutoffs proposed in younger patients (Chao et al. 2013). The NCCN guidelines designate CA-125 as an optional test in both workup and surveillance, while the American Society of Gynecologists Oncologists does not endorse the routine use of CA-125 during surveillance in the absence of clinical findings concerning for metastatic disease (Salani et al. 2011). Future studies regarding the use of CA-125 are warranted and will likely focus on its potential as a tool for prediction of extrauterine disease in early stage patients or its use during surveillance for early detection of disease recurrence and whether this translates to improved patient outcomes.
3.4 Histologic Factors
While tumor stage is the most important prognostic indicator, many of the other confirmed prognostic features relate to information from the histology of the tumor itself. Tumor cell type, grade of differentiation, and LVSI are significantly important, and assist with stratification of patients within surgical staging groups into risk categories. Thus, the results of each can have significant influence on the adjuvant therapy given, as patients with early stage, low risk histology may not require adjuvant therapy, the same stage patient with high risk histology or tumor grade may have poorer outcomes if adjuvant therapy is not offered.
3.4.1 Histology
Given that surgical staging predominates for endometrial cancer, characteristics found on pathologic evaluation are highly prognostic. Cell type and tumor grade are highly predictive of patient outcomes, and carry significant weight in determining if adjuvant therapy after hysterectomy should be offered. Additional information regarding myometrial invasion, cervical stromal invasion, lymphovascular invasion, and others have also been shown to be prognostic and are used to help stratify risk of recurrence in patients with early stage disease. The following section on histology relates to histologic factors studied largely in endometrioid adenocarcinomas. In general, non-endometrioid histologies such as papillary serous and clear cell adenocarcionoma are highly correlated with many of these adverse pathologic factors, thus are deemed high risk in even early stage disease, and are offered more aggressive adjuvant therapy.
3.4.1.1 Histologic Type
The vast majority of endometrial cancers arise within the endometrial layer of the uterus, with subsequent growth and spread, usually into the myometrium, as it progresses. Adenocarcinoma accounts for the majority of endometrial cancer cases diagnosed. The most common histologic subtype is endometrioid histology, accounting for nearly 75–80 % of endometrial cancer cases. This is a gland forming variant of adenocarcinoma, often with appearance similar to that of the endometrium. Overall prognosis for low grade endometrioid adenocarcinoma is favorable. By some reports, approximately 25 % of adenocarcinomas can have squamous differentiation, where the grade of the glandular component is prognostic (Abeler and Kjorstad 1992). Villoglandular and mucinous adenocarcinomas are infrequently identified, with no significant effect on outcomes with villoglandular (Zaino et al. 1998a), and improved outcomes with mucinous features (Ross et al. 1983). Two less common, yet clinically significant subsets of adenocarcinoma, include papillary serous and clear cell adenocarcinoma, accounting for a majority of the remaining non-endometrioid cases. Papillary serous carcinomas histologically have a complex papillary architecture, resembling serous carcinoma of the ovary. Nuclear atypia is common, and psammoma bodies can be present. Clear cell carcinomas have 3 types of growth patterns, tubulocystic, papillary, or solid patterns, and are less likely to contain psammoma bodies. Any tumor that contains 10 % or more of either papillary serous or clear cell adenocarcinoma features are classified as mixed histology, although prognosis tends to correlate with the most advanced histology in the tumor.
Endometrial cancer is subdivided into type 1 or type 2 tumors; type 1 defined as low grade (FIGO grade 1 and 2) endometrioid tumors (nearly 80 % of adenocarcinoma), and type 2 encompassing FIGO grade 3 endometroid tumors, papillary serous, and clear cell adenocarcinomas. A different etiology of tumorigenesis has been proposed in these two subgroups. Type 1 tumors are generally associated with the classical risk factors for endometrial cancer including nulliparity, obesity, unopposed estrogen, early menarche/late menopause, tamoxifen therapy, among others. It has been proposed that elevated estrogenic state experienced in these situations can stimulate the endometrial layer, leading to hyperplasia, a likely precursor to endometrial cancer in some settings. Type 2 tumors, on the other hand, are not associated with hyperestrogenism or endometrial hyperplasia. Stage by stage, more aggressive histology is associated with poorer clinical outcomes (Boruta et al. 2004). As such, type 2 tumors are often included as a risk factor warranting intensification of adjuvant therapy as discussed below.
Uterine sarcomas (endometrial stromal sarcomas, leiomyosarcomas, and other mesenchymal tumors), and mixed epithelial and mesenchymal tumors (adenosarcomas and malignant mixed mullerian tumors), are much less common types of uterine cancer. As a group, they all confer very poor prognosis at diagnosis. They tend to be associated with higher stage at diagnosis, and dismal disease free and overall survival (Prat 2009; Callister et al. 2004). More aggressive therapy is generally favored in this group of patients given their significantly higher risk for failure and death, however given the relative rarity, poor response to proposed interventions, and paucity of prospective data, there is no clearly defined guideline in management (Rauh-Hain and Del Carmen 2013; Kanthan and Senger 2011).
3.4.1.2 Tumor Grade
Across a multitude of studies, tumor grade has been shown to be strongly associated with prognosis, degree of myometrial invasion, and risk for lymph node metastasis. FIGO grading of endometrioid carcinomas incorporate the degree of gland formation and nuclear grade. The percent solid (nonglandular) growth is scored as increased solid growth is associated with more aggressive behavior. Grade 1 is defined as no more than 5 % solid growth, grade 2 with 6 to 50 percent solid growth, and grade 3 with more than 50 percent solid growth. If glandular grade is different from nuclear grade, nuclear grade predominates. Non-endometrioid tumors are graded by nuclear grade alone. Zaino et al. reported 5-year survival rates of 94 % for grade 1, 84 % for grade 2, and 72 % for grade 3 tumors (Zaino et al. 1998a). Given the significant prognostic feature of tumor grade, it is incorporated into risk stratification of patients within a given stage to help direct adjuvant therapy.
3.4.1.3 Myometrial Invasion
Degree of myometrial invasion has been shown to be an independent predictor for outcome in a multitude of studies (Creasman et al. 1987; Morrow et al. 1991). This has been validated since originally described and continues to be incorporated as part of the current FIGO staging. While risk factor groups have been described based on thirds of invasion, the most recent revision of FIGO staging has established 50 % as the cutoff between stage IA and stage IB endometrial cancer.
3.4.1.4 Cervical Stromal Invasion
Cervical stromal invasion is included in FIGO staging, given its prognostic significance in outcomes with reduced 5-year disease-free survival of 74–80 % for stage II disease compared to 90–96 % for stage IA. Previously, any cervical invasion was classified as stage II disease in the 1988 FIGO schema, with stage IIA defined as isolated endocervical epithelial involvement and stage IIB for deeper stromal invasion. However, several reports failed to demonstrate a difference in survival between the two groups (Orezzoli et al. 2009; Eltabbakh and Moore 1999). Thus, this subclassification was eliminated with the recent 2009 revision of FIGO staging and currently cervical stromal invasion only constitutes stage II disease. This has been shown to be independently prognostic for patient outcomes, with a 44 % increase in risk of progression or death and a 33 % increase in risk of death (Tewari et al. 2012).
3.4.1.5 Lymphovascular Space Invasion
Lymphovascular space invasion (LVSI) has been shown to be a predictor of risk of relapse and poorer survival, independent from tumor grade or depth of myometrial involvement (Morrow et al. 1991; Mariani et al. 2002b, c). LVSI has been shown to increase the rate of pelvic lymph node metastasis (Creasman et al. 1987). LVSI continues to be used as one of several histologic criteria for risk stratification for adjuvant therapy selection and clinical trial inclusion.
3.4.1.6 Peritoneal Cytology
In previous 1988 FIGO staging, the presence of malignant cells in peritoneal fluid was designated stage IIIA disease. However, multiple studies failed to show this as an independent prognostic factor (Hirai et al. 1989; Tebeu et al. 2004; Takeshima et al. 2001). The revised 2009 FIGO staging has eliminated positive peritoneal cytology as a factor in staging. However, recently Milgrom et al. showed that in stage III patients, positive peritoneal cytology was predictive of outcome and associated with distant relapse (Milgrom et al. 2013). This is consistent with the observation that positive peritoneal cytology, while not independently prognostic, may enhance the negative impact of other adverse factors (Takeshima et al. 2001). Peritoneal cytology is still obtained at most institutions during hysterectomy, as it may have some effect on adjuvant therapy selection, and is used as inclusion criteria of some ongoing phase III trials.
3.4.2 Implications of Postoperative Histology on Adjuvant Treatment
As previously discussed, multiple histologic and clinical factors have been found to be independently prognostic of clinical outcome. While some of these are directly used for staging, others are used for risk stratification to help predict a benefit from adjuvant therapy and aid in the selection of adjuvant therapy.
3.4.2.1 Risk Group Stratification in Early Stage Endometrial Cancer
Adjuvant therapy in endometrial cancer is dictated in large part by stage and risk factors within each stage. This is specifically true for early stage endometrial cancer where the extent and method of adjuvant radiotherapy has evolved.
Observation is reasonable for patients with stage IA, grade 1, favorable histology disease, otherwise deemed low risk. In patients with stage I disease, and any risk factor, including Grade 2–3 disease, LVSI, lower uterine segment involvement, deep myometrial invasion, or advanced age > 50–70, adjuvant therapy has traditionally been considered. Previously, the GOG 33 data demonstrated advanced grade or deep myometrial invasion were risk factors for lymph node positive disease, which was associated with worse disease free survival. These risk factors had been employed to determine the need for postoperative pelvic radiation, but how to much weight to assign these risk factors has evolved.
The traditional indications for pelvic radiation in early stage disease have been challenged by the results of the Post-Operative Radiation Therapy in Endometrial Cancer (PORTEC) (Creutzberg et al. 2003, 2004) and GOG 99 (Keys et al. 2004) studies, resulting in identification of a new set of risk factors. This paradigm change has also been fueled by advances in surgical approach over the past 2 decades, with a more comprehensive degree of lymph node dissection, even in co-morbid patients. Based on both trials’ results, a new high-intermediate risk group was defined by each cooperative group and a more multi-faceted algorithm was developed that incorporated grade, depth of myometrial invasion, LVSI, and age. PORTEC’s and GOG 99’s results are highly consistent showing an incidence of failure in the 30 % range for the GOG-defined high-intermediate-risk group and for the grade 3 group in PORTEC. The high-intermediate-risk group was defined by GOG as (1) grade 2–3 with deep third myometrial invasion and LVSI; or (2) age > 50 and two of the risk factors in (1); or (3) age > 70 and one of the risk factors in (1). The definition based on the PORTEC data is similar: <50 % myometrial invasion and grade 3 (any age); or >50 % invasion and grade 1–2 and age > 60 years. However, the combination of <50 % myometrial invasion, grade 3 and LVSI is considered a high-risk feature by PORTEC-2 due to the significantly lower 5-year overall survival of 58 % observed in the PORTEC 1 study (Creutzberg et al. et al. 2004). These overall results are supported by a metaanalysis by Kong et al. (2007) of all four randomized trials (Creutzberg et al. 2000, 2004; Keys et al. 2004; Aalders et al. 1980) that shows adjuvant radiotherapy improved disease specific and overall survival for patients with grade 3 tumors and stage IB (>50 % invasion) disease. The failure pattern in the high-intermediate-risk group has been found to consist largely of vaginal recurrences, therefore, while the high risk patients are often recommended pelvic radiotherapy, high-intermediate risk group patients are often offered vaginal cuff brachytherapy and/or pelvic radiotherapy as vaginal recurrences are the most likely site of failure. This group has been studied by PORTEC-2, and vaginal cuff brachytherapy was found to be equivalent in preventing pelvic recurrence to whole pelvic radiation (Nout et al. 2010).
Adjuvant therapy for high risk disease is an area of active research as there is data to suggest intensifying therapy with chemotherapy is warranted, and currently practiced at many institutions. PORTEC-3 is currently enrolling patients with the high risk criteria and randomizing patients postoperatively to pelvic radiation or pelvic radiation with concurrent and post-radiation chemotherapy. Eligible patients include those with <50 % myometrial invasion plus grade 3 and LVSI; >50 % myometrial invasion with grade 3, or advanced endometrial cancer, including stage II–III disease, papillary serous or clear cell histologies. The results are eagerly anticipated.
3.4.2.2 Locally Advanced Endometrial Cancer
Stage III and IVA endometrial cancer is often described as locally advanced endometrial cancer. This group represents a heterogenous group of patients, with varying degrees of tumor burden and tumor histology, with the best adjuvant therapy not clearly defined. GOG 122 established a role for chemotherapy over whole abdominal radiation owing to improved disease free and overall survival of 38–50 %, and 42–55 %, respectively (Randall et al. 2006). More recently, Hogberg et al. compiled the data from two randomized European trials, NSGO-EC-9501/EORTC-55991 and MaNGO ILIADE-III, which randomized patients to adjuvant radiotherapy alone or sequential chemotherapy and radiation therapy. This indicated a significant improvement in 5-year progression-free survival from 69 to 78 %, with a trend for improved overall survival (Hogberg et al. 2010). The extent of radiotherapy, timing with chemotherapy, and patient selection is still an area of active study.
3.4.3 Molecular Markers
Molecular markers are an area of active interest. In most cases, markers are correlated with established prognostic indicators, such as tumor histology and grade. Some of the most studied factors are briefly reviewed. To date, the clinical utility of these markers is limited.
3.4.3.1 DNA Ploidy
DNA content, or more specifically, aneuploidy, has been studied by many groups. The frequency of aneuploidy has been shown to increase with increased tumor grade (Lundgren et al. 2002). Papillary serous carcinoma has been shown to exhibit aneuploidy, as well (Prat et al. 1994). Further, DNA aneuploidy has been shown to be an independent predictor for disease free survival (Zaino et al. 1998b; Nordstrom et al. 1996).
3.4.3.2 Microsatellite Instability
Microsatellite instability (MSI) is strongly associated with endometrial cancer in patient with HNPCC, occurring in nearly 75 % of such patients, and occurs in approximately 25–45 % of sporadic endometrial carcinomas. Microsatellites are short repeats of DNA that are integrated throughout the genome, and MSI is associated with deficits in DNA mismatch repair. In some studies, MSI is associated with improved clinical outcome (Maxwell et al. 2001). However, there is discrepancy in the published literature, with several reports showing no correlation with clinical outcome (Zighelboim et al. 2007; Baldinu et al. 2002), while others have shown MSI to be an independent prognostic indicator for poorer survival (Mackay et al. 2010; Nout et al. 2012; Steinbakk et al. 2011). This disagreement may be related to sample size, cohort selection, different adjuvant therapies, confounding variables, or may indicate identification of the specific downstream genetic alterations is actually more relevant (Steinbakk et al. 2011).
3.4.3.3 Ki-67 Proliferation Index
Cellular proliferation is an area of interest in most cancer cell types. This has also been evaluated by many groups for endometrial cancer. Nuclear Ki-67 antigen is a marker of proliferating cells, and has been shown to be associated with histological grade and depth of myometrial invasion, as well as other risk factors (Kudela et al. 2012). High levels of Ki-67 expression have also been associated with increased risk of recurrence and poorer survival in some studies (Salvesen et al. 1998).
3.4.3.4 Oncogenes
HER2 and EGFR are both members of the ErbB/HER signaling family, a group of tyrosine kinase receptors critical in cellular proliferation and differentiation, and are implicated in tumorigenesis in many tumor models. HER2 expression was associated with higher tumor grade and depth of myometrial invasion but not independently prognostic for survival, whereas EGFR overexpression in endometrioid adenocarcinoma decreased survival from 89 to 69 % (p < 0.04), and in serous papillary and clear cell from 86 to 27 % (p < 0.03) (Khalifa et al. 1994; Konecny et al. 2009). There is continued interest in this pathway as inhibitors of EGFR and HER2 are actively used in other cancer treatment and exploitation of this pathway with these pharmaceuticals theoretically may improve patient outcomes.
P53 has been reported to be more highly expressed in type 2 tumors (Kudela et al. 2012). Not surprisingly, this has also been correlated with poorer patient outcomes (Mariani et al. 2000; Saffari et al. 2005; Silverman et al. 2000). Currently, clinical utility of this marker is uncertain as no targeted therapies are readily available.
The evaluation of PTEN as a prognostic factor is also controversial. PTEN is a tumor suppressor gene that down regulates the PI3-Kinase pathway, thus slowing down cellular proliferation. PTEN is mutated in approximately 20–80 % of endometrial cancers, but with less frequency in serous carcinoma. Results regarding the effect of PTEN on patient outcomes is mixed (Latta and Chapman 2002).
3.4.3.5 Cell Adhesion Molecules
Cell adhesion molecules have been widely studied in tumor biology, and are responsible in part for coordinating cell–cell interaction, cellular proliferation, and metastasis. E-cadherin is a cell membrane protein that complexes with cytoplasmic B-catenin regulating cellular adhesion and growth. The loss of E-cadherin expression results in release of B-catenin, which is then able to induce a subset of genes responsible for endothelial to mesenchymal transition which is one mechanism by which tumorigenesis and metastasis is thought to occur. Loss of E-cadherin expression is commonly seen in non-endometrioid endometrial carcinoma, but occasionally in endometrioid histology (Holcomb et al. 2002; Mell et al. 2004). Although in the same pathway, B-catenin has not been found to be independently prognostic of clinical outcomes (Nout et al. 2012; Singh et al. 2011).
3.4.3.6 Steroid Receptors
Expression of estrogen receptor (ER) and progesterone receptor (PR) has been extensively examined, given hormonally directed therapy is of particular interest in patients who may not be surgical candidates or have otherwise limited treatment options. Some studies indicate ER and PR expression are associated with less aggressive tumor behavior/grade (Ferrandina et al. 2005; Geisinger et al. 1986; Kadar et al. 1993; Jeon et al. 2006). While progestins are often used in relapsed or advanced disease, a recent metaanalysis indicates there is no data at present to support its use in primary disease (Martin-Hirsch et al. 2011); prospective evaluation of receptor expression and treatment response is warranted.
3.5 Imaging Prognostic Factors
FIGO staging for endometrial cancer by definition requires surgical staging. In the United States, a majority of centers include routine pelvic lymphadenectomy and para-aortic lymph node sampling at the time of hysterectomy. Morbidity is associated with such extended surgery, although has improved with advances in surgical technology. Further, the ASTEC trial, albeit with relatively limited follow-up, to date has not shown a survival benefit to lymphadenectomy in early stage disease (ASTEC study group et al. 2009). Thus, there is great interest in developing new ways to predict risk of lymph node involvement, and to identify those patients with acceptably low risk of involvement in order to identify patients where omission of lymphadenectomy is reasonable. While clinical exam prior to 1988 was shown to understage endometrial cancer in 13–22 % of patients, newer imaging technology is now available, and may be promising in identification of factors such as myometrial invasion, extrauterine involvement, as well as risk of pelvic lymph node disease. These are briefly reviewed here.
3.5.1 Morphologic Imaging
Computed tomography (CT) has been used for preoperative assessment in endometrial cancer, but its role is with limitations. The ability of CT to delineate endometrial cancer in the uterus is relatively insensitive, especially for small endometrial cancers (i.e. stage IA), with overall sensitivity of 53 % (Grossman et al. 2008). Accuracy of CT for myometrial invasion has been reported to be 61 % with sensitivity of 40 % in one study comparing ultrasound, CT, and MRI for depth of myometrial invasion assessment (Kim et al. 1995). Multidetector CT has improved accuracy for depth of myometrial invasion and cervical involvement at 95 and 81 %, respectively (Tsili et al. 2008). The applicability of this modality is limited given this single experience in 16 patients, thus warrants further evaluation. Sensitivity and specificity of CT for lymph node involvement has been reported at 52 and 92 %, respectively (Connor et al. 2000). Chest CT can be considered in high risk patients, such as advanced stage or high grade tumors who are at increased risk for pulmonary metastasis.
The accuracy of ultrasound for myometrial invasion has been described by many groups. The accuracy of transvaginal ultrasound (TVUS) for predicting stage IA versus stage IB endometrial cancer reportedly ranges from 69 to 93 % (Kim et al. 1995; DelMaschio et al. 1993; Prompeler et al. 1994). High-frequency TVUS has been shown to have accuracy of 73 % for assessment of myometrial invasion (Arko and Takac 2000). The reported experience of ultrasonography to predict cervical involvement has also been limited, with only 7 of 10 patients with pathologic cervical involvement reported pretherapy to have involvement based on ultrasound (Akbayir et al. 2011; Szantho et al. 2001). The use of 3D ultrasonography with volume contrast imaging has also been described. Jantarasaengaram et al. reported accuracy of 92 % for predicting myometrial invasion and 90 % for cervical involvement (Jantarasaengaram et al. 2013). Sonohysterography, which involves intracavitary infusion of saline followed by evaluation with TVUS, has been employed in some settings, with accuracies of 84–89 % for assessing deep myometrial invasion (Chang et al. 2010; Valenzano et al. 2001; Dessole et al. 2006). The use of this modality is controversial, however, due to concern of tumor spillage into the peritoneal cavity with saline infusion, which has been documented by some investigators (Dessole et al. 2006; Alcazar et al. 2000).
The use of ultrasound has been compared to MRI in multiple investigations, and consistently has been found to be superior to ultrasound for evaluation of cervical involvement and depth of myometrial invasion (Kim et al. 1995; DelMaschio et al. 1993; Arko and Takac 2000; Antonsen et al. 2013a; Yamashita et al. 1993a). Further, contrast enhanced MRI, compared to unenhanced MRI, results in significantly improved accuracy, ranging from 85 to 92 % accuracy for depth of myometrial invasion versus 55–78 % for non-contrasted imaging (Kinkel et al. 1999; Ito et al. 1994; Saez et al. 2000; Sironi et al. 1992; Yamashita et al. 1993b; Sala et al. 2009). Accuracy rates for determination of cervical involvement range from 86 to 95 % (Manfredi et al. 2004; Takahashi et al. 1995; Nagar et al. 2006). The use of MRI for pelvic and para-aortic lymph node involvement is comparable to CT, with sensitivity and specificity reported at 44–66 % and 73–98 %, respectively. Thus, given MRI’s superior assessment of depth of myometrial invasion and cervical involvement, it is generally preferred over CT and ultrasound for preoperative workup.
3.5.2 Functional Imaging
The use of PET/CT in endometrial cancer is an area of active investigation. A recent meta-analysis of 18F-FDG PET or PET/CT for identification of metastatic lymph nodes in endometrial cancer reported the pooled estimates for 243 patients, indicating sensitivity and specificity of 63 % (95 % CI, 48.7–75.7 %) and 94.7 % (95 % CI, 90.4–97.4 %), respectively (Chang et al. 2012). The relatively low sensitivity is uncertain, but may be related to low glucose metabolism in low grade lesions, as well as limited ability to detect subcentimeter metastases. Further, PET imaging is limited in ability to detect intraperitoneal tumor implants and parenchymal implants. Due to these limitations, CT and MRI are preferable for detection of extrauterine disease, although FDG-PET may be appropriate in patients with high grade tumor that is likely to be FDG avid (Lee et al. 2011).
The role for PET/CT for assessment of myometrial invasion and cervical invasion is uncertain. Antonsen et al. recently reported the results of 318 patients with endometrial cancer who preoperatively underwent 2D ultrasonography, MRI, and PET/CT imaging. Sensitivity, specificity, and accuracy for PET/CT for myometrial invasion were 93, 49, and 61 %, and 43, 94, and 83 %, respectively for cervical invasion, which were similar to MRI (Antonsen et al. 2013a).
SUVmax has been evaluated by some groups, with limited data suggesting SUVmax may be able to predict higher stage disease, higher grade tumors, risk of deeper myometrial invasion, and lymph node metastatic risk (Antonsen et al. 2013b; Nakamura et al. 2010). Other studies have indicated SUVmax can also predict for poor disease free survival (Kitajima et al. 2012) and overall survival (Nakamura et al. 2011, 2013).
Finally, 18F-FDG PET or PET/CT has also been used for detection of recurrent disease (Park et al. 2008; Belhocine et al. 2002; Chung et al. 2008; Kitajima et al. 2008). Saga et al. assessed the use of 18F-FDG PET in 21 patients for detection of recurrence and evaluation of treatment response. Compared to conventional imaging and serum tumor markers, FDG-PET combined with CT or MRI was more accurate and had comparable or better sensitivity and specificity (Saga et al. 2003). Currently, the ACR guidelines indicate that FDG-PET is usually appropriate over MRI pelvis or CT pelvis if recurrence is suspected clinically (Lee et al. 2011).
4 Cancer of the Vulva
Vulvar cancer is a rare disease, accounting for only 5 % of malignancies of the female genital tract (Siegel et al. 2012). It is estimated that in 2013 there will be approximately 4,700 new cases and 900 deaths due to this disease in the United States (Siegel et al. 2012). The mean age at diagnosis for vulvar cancer is 65 years, and clinical risk factors for this disease include immunodeficiency, prior history of cervical cancer, cigarette smoking, vulvar dystrophy, vulvar or cervical intraepithelial neoplasia, and HPV infection (Ansink 1996; Madsen et al. 2008).
Vulvar cancer is a disease of the skin, arising from squamous epithelium, and tumor spread occurs primarily through the lymphatic system. The first station of nodal spread is the inguino-femoral lymph nodes, usually superficial first then deep, which then spreads in a predictable fashion to the pelvic lymph nodes in more advanced cases. Pelvic lymph node involvement without inguinal node involvement is rare (Krupp and Bohm 1978). Locally, vulvar cancer can invade adjacent structures including the vagina, bladder, anus and rectum. Given the propensity of this type of cancer to spread to adjacent structures and metastasize to lymph nodes, standard of care had previously been en bloc resection of the primary tumor with inguinofemoral lymph node dissection, resulting in significant risk of morbidity and psychosexual impact. However, the approach to treatment has evolved over the last several decades, with therapy ranging from wide local excision for small, superficial lesions, to definitive or neoadjuvant chemo-radiation which may reduce the extent of surgical resection required, versus pelvic exenteration in advanced disease.
4.1 Staging
Prognostic factors for vulvar cancer include size and local extension of the primary tumor, as well as the degree of lymphatic involvement, as reflected in the most recent (2009




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree