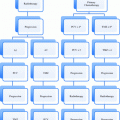
Stage
Description
Primary tumor (T)
TX
Primary tumor cannot be assessed
TO
No evidence of primary tumor
Tis
Carcinoma in situ: intraepithelial tumor without invasion of the lamina propria
T1a
Tumor invades lamina propria or muscularis mucosae
T1b
Tumor invades submucosa
T2
Tumor invades muscularis propriaa
T3
Tumor penetrates subserosal connective tissue without invasion of visceral peritoneumb or adjacent structures (including spleen, transverse colon, liver, diaphragm, pancreas, abdominal wall, adrenal gland, kidney, small intestine, and retroperitoneum)
T4a
Tumor invades serosa (visceral peritoneum)
T4b
Tumor invades adjacent structures
Regional lymph nodes (N)
NX
Regional lymph nodes cannot be assessed
N0
No regional lymph node metastasis (pN0 denotes negative finding in all examined lymph nodes, regardless of the total number removed and examined)
N1
Metastasis in 1–2 regional lymph nodes
N2
Metastasis in 3–6 regional lymph nodes
N3a
Metastasis in 7–15 regional lymph nodes
N3b
Metastasis in ≥16 regional lymph nodes
Distant metastasis (M)
M0
No distant metastasis
M1
Distant metastasis (including seeding of the peritoneum and positive peritoneal cytology)
2.2 Nomograms
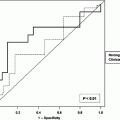
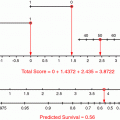
Selecting patients for adjuvant therapy based solely on AJCC stage is not entirely accurate. The AJCC stage groupings stratify disease-specific survival after an R0 resection into risk groups based on the depth of tumour invasion and the number of positive lymph nodes. All patients grouped within a particular AJCC stage group are assumed to have the same prognosis and are therefore treated in the same way. However, for individual patients, risk can vary substantially within a particular stage group depending on other prognostic factors. Tools for individual patient prognostication have been developed that outperform the AJCC classification in prognostic accuracy. In 2003, Kattan et al. from MSKCC developed a postoperative nomogram for predicting disease-specific survival after an R0 resection for gastric cancer (Kattan et al. 2003) (Fig. 1). Rather than stratifying patients into risk groups, nomograms are tools that combine all proven prognostic factors to predict individual patient risk. The MSKCC nomogram utilised data from 1,039 patients who had undergone R0 resections. Nomogram predictor variables included age, sex, primary tumour site, Lauren histology, number of positive lymph nodes, number of negative lymph nodes, and depth of invasion. The concordance index for the model was 0.80. The concordance index provides the probability that for two randomly selected patients in which one patient has an event before the other, the patient who had the event first has a poorer predicted outcome from the nomogram (for concordance, higher is better). When compared with the predictive ability of AJCC stage, the nomogram discrimination was superior (concordance index 0.80 versus 0.77; P < 0.001). The authors noted heterogeneity within several of the AJCC stage groups with a range of nomogram-predicted probabilities within each group.
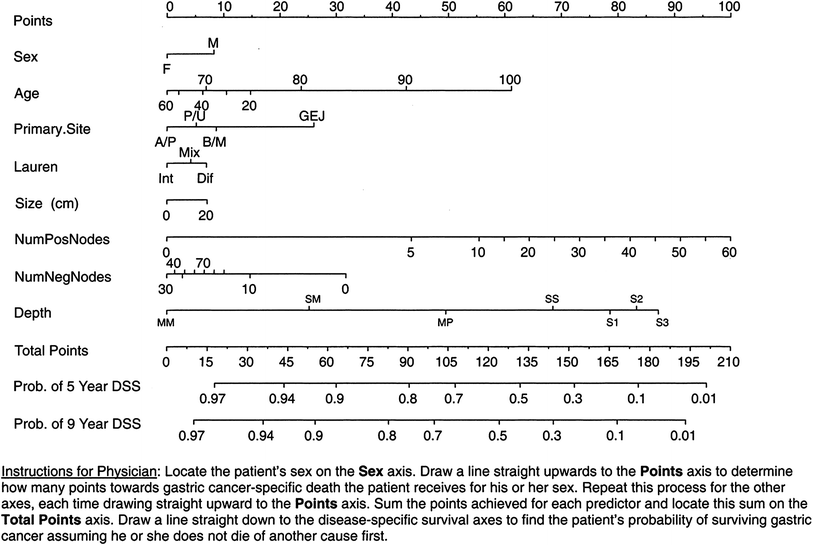

Fig. 1
Memorial Sloan-Kettering Cancer Center nomogram for disease-specific survival (DSS). Number of positive nodes (NumPosNodes); number of negative nodes (NumNegNodes) probability (Prob.); antrum or pyloric (A/P), body or middle one-third (B/M), gastroesophageal junction (GEJ); proximal or upper one-third (P/U); intestinal (int); mixed (mix); diffuse (Dif); mucosa (MM); propria muscularis (MP); suspected serosal invasion (S1); definite serosal invasion (S2); adjacent organ involvement (S3); submucosa (SM); and subserosa (SS). Reprinted with permission © (2003) American Society of Clinical Oncology. All rights reserved. Kattan et al. (2003)
In the last 10 years, D2 lymph node dissection has become more commonly practiced worldwide despite the fact that published randomised trials have not demonstrated improved survival with more extensive nodal dissections (Bonenkamp et al. 1999; Cuschieri et al. 1999). One of the main criticisms of the INT0116 trial has been the lack of quality assurance relating to surgical technique. It has been claimed that the benefits of chemoradiation are only due to the compensation of poor surgery, and that these benefits would not be seen if a D2 dissection had been performed. D2 nodal dissection has been the standard of care in Eastern countries for many decades and the survival rates for Eastern patients with gastric cancer are consistently higher than those for Western patients (MacDonald 2011; Strong et al. 2010). A nomogram predicting 5- and 10 year overall survival after D2 gastrectomy in Eastern patients has recently been reported (Han et al. 2012). Han et al. analysed data from 7,954 patients who underwent D2 gastrectomy at Seoul National University Hospital (SNUH) in Korea. The nomogram was constructed and validated by randomly assigning two-thirds of the patients to the training set (n = 5,300) and one-third to the validation set (n = 2,654). Nomogram predictor variables included age, sex, primary tumour location, depth of invasion, number of metastatic lymph nodes, and number of examined lymph nodes. The nomogram was validated firstly using the SNUH validation set and then an additional external validation was performed using a data set (n = 2,500) from the Cancer Institute Ariake Hospital (CIAH) in Tokyo, Japan. In the SNUH validation set, the actual survival corresponded closely with the predicted survival, and the nomogram exhibited superior discrimination power compared with the current 7th edition of the AJCC staging system for gastric cancer (concordance index 0.78 versus 0.69; P < 0.001). Similar to the MSKCC nomogram, a wide range of predicted survivals could be identified within each AJCC stage group. In the CIAH validation set, the concordance index was 0.79 and the predicted survival was within a 10 % margin of nomogram prediction.
The use of a nomogram can improve prognostic accuracy when trying to predict outcome after surgery in an individual patient. The information derived can be used to decide whether adjuvant treatment is required if the predicted risk of treatment failure exceeds some predefined threshold. For generalised use of a nomogram by other institutions or other countries, it is important to minimise the effect of differences in surgical technique and pathologic examination. Details regarding D2 gastrectomy and the minimum number of nodes examined are not provided in the report by Kattan. Nevertheless, the MSKCC nomogram has been externally validated for accuracy in several Western patient cohorts (Koc et al. 2009; Novotny et al. 2006; Peeters et al. 2005). In the SNUH data set, all patients underwent a D2 gastrectomy and patients were excluded if the number of examined lymph nodes was less than 16. However, although this nomogram may be useful in Korea and Japan where D2 gastrectomy is routinely performed, it has not been validated using a Western patient cohort. The universal applicability of these nomograms across both Eastern and Western populations remains a question for several reasons. Firstly, the treatment paradigms for adjuvant therapy are different in the East and West. In Eastern countries, adjuvant therapy consists almost exclusively of postoperative chemotherapy, and there is very little use of radiotherapy or preoperative treatment (Bang et al. 2012; Sakuramoto et al. 2007). Secondly, there are clear differences in the epidemiology and clinical presentation of gastric cancer in the East compared with the West (Bunt et al. 1995; Guggenheim and Shah 2012; Verdecchia et al. 2003). Some studies also suggest that Eastern patients may have better survival rates, stage-for-stage compared with Western patients, even after controlling for the extent of surgical resection (Strong et al. 2010).
2.3 Combining Postoperative Chemoradiation with Preoperative Chemotherapy
One dilemma faced by clinicians is how to manage patients who commence treatment with preoperative ECF but show no evidence of tumour downstaging at surgery. Should these patients continue with postoperative ECF according to the MAGIC regimen, or should they change to postoperative chemoradiation? This question is being addressed in the ongoing CRITICS trial that is being conducted in the Netherlands and Scandinavia. CRITICS is a randomised trial of preoperative epirubicin, cisplatin and capecitabine [ECX] followed by either surgery and additional ECX (i.e., MAGIC), or by surgery and postoperative chemoradiation (i.e., INT0116). The question being addressed by this trial is whether postoperative chemoradiation improves survival and/or locoregional control compared to postoperative chemotherapy alone in patients that receive preoperative chemotherapy followed by surgery. Currently, in the absence of any randomised data to guide treatment decisions, it is not unreasonable to offer postoperative chemoradiation to those patients who have not demonstrated any evidence of downstaging with preoperative chemotherapy (i.e., those with pathological T3-4 tumours or node positive tumours). It is known from the INT0116 trial that postoperative chemoradiation is effective in these patients, while the contribution of postoperative chemotherapy as given in the MAGIC trial is controversial given that only 42 % of patients completed the postoperative chemotherapy (Macdonald et al. 2001).
2.4 Molecular Biomarkers
The development of new molecular biomarkers can be used to identify subgroups of tumours with different biological and clinical behavior. Several molecular techniques have been utilized to identify potential predictors of lymph node status in patients with gastric cancer including altered DNA copy number and expression of specific genes, genome wide mRNA expression analysis, and gene expression profiling (Buffart et al. 2009; Li et al. 2008; Marchet et al. 2007; Teramoto et al. 2005; Wu et al. 2008; Zheng et al. 2006). However, none of the identified markers have shown clinical utility and the gold standard to predict prognosis after surgery remains TNM staging.
3 Prognostic and Predictive Markers Associated with Chemoradiotherapy
3.1 Clinico-Pathological Features
3.1.1 Postoperative Chemoradiotherapy
There is very little information in the published literature reporting prognostic factors for survival after chemoradiation for gastric cancer. The 5 year survival estimates for patients treated on INT0116 show decreasing survival with increasing T-stage and number of positive lymph nodes (MacDonald 2011). Patients with T1-2 tumours had 56 % 5 year survival compared to 38 % for patients with T3 tumours. The 5 year survival estimates for patients with N0, N1-3, and N > 4 nodal disease were 60, 50 and 30 % respectively. In a recent update of the INT0116 trial, Smalley et al. performed exploratory subset analyses of treatment effect for the following variables; sex, race, T-stage, N-stage, D level of resection, primary tumour location, and histology (Smalley et al. 2012). These analyses showed that postoperative chemoradiation was beneficial in all subsets with the exception of patients with diffuse histology who exhibited minimal non-significant treatment effect. The authors were unable to explain this finding and caution that it may be a random observation of an unplanned subset analysis.
Several studies have shown lymph node status to be an important prognostic factor for survival following adjuvant chemoradiation. Quero et al. have recently reported a series of 52 patients who underwent gastrectomy and postoperative chemoradiotherapy for gastric cancer (Quero et al. 2012). The proportion of patients who had D1 and D2 lymph node dissection was 71 and 15 % respectively. This study reported pathologic nodal status to be predictive of disease-free survival. Patients with ≤6 positive lymph nodes had a 5-year disease-free survival rate of 57 % compared to 32 % for patients with 7–15 positive nodes and 18 % for patients with >15 positive nodes (p = 0.02). Osti et al. reported a series of 55 patients with gastric cancer who underwent D2 gastrectomy followed by postoperative chemoradiotherapy (Osti et al. 2012). In this study, lymph node ratio (LNR: defined as the ratio of the number of metastatic nodes to the number of removed nodes) ≥4 and N3 nodal stage were significant prognostic factors for overall survival and relapse. In addition, stage III–IV disease was also identified as being a significant prognostic factor for survival. Chang and co-workers treated a series of 120 patients with gastric cancer over a 10 year period using the INT0116 regimen (Chang et al. 2011). Fifty-two percent of patients underwent conventional radiotherapy planning and were treated with simple AP-PA techniques, while 48 % underwent CT planning. This study showed that stage of disease, and particularly nodal stage was an important prognostic factor for survival. Interestingly, the authors also reported that CT planning was a favourable predictor of survival as compared with conventional planning. Kassam et al. from the Princess Margaret Hospital reported a series of 82 patients with resected gastric cancer who were treated with postoperative chemoradiotherapy (Kassam et al. 2006). Only 6.1 % of patients underwent a D2 lymph node dissection, while 48 % underwent less than a D1 dissection. In this series, tumour and nodal stage significantly influenced relapse-free and overall survival. Three year relapse-free survival was 74 % for patients with T1/2 tumours compared to 36 % for patients with T3/4 tumours (p = 0.008). Three year overall survival was 85 % for T1/2 tumours versus 53 % for T3/4 tumours (p = 0.02). Patients with N0/1 tumours had a 3-year relapse-free survival of 63 % compared to 0 % for patients with N2/3 tumours (p < 0.001). Three year overall survival was 80 % for N0/1 tumours versus 0 % for N2/3 tumours (p < 0.001). Zhu et al. have recently reported the results of a randomised, multicentre trial from China that compared IMRT plus concurrent chemotherapy with chemotherapy alone in 380 gastric cancer patients treated with D2 gastrectomy (Zhu et al. 2012). IMRT was associated with an increase in the median duration of relapse-free survival (50 months for IMRT versus 32 months for chemotherapy alone; p = 0.029), although there was no significant difference in overall survival. An analysis of prognostic factors showed that lymph node metastasis and TNM stage were both independent prognostic factors. This study provides the first phase III evidence of benefit for postoperative chemoradiation in patients undergoing D2 gastrectomy.
Verheij et al. have recently assessed the performance of the MSKCC gastric nomogram in a cohort of 139 patients who received postoperative chemoradiation after an R0 resection for gastric cancer (Verheij et al. 2012). As previously described, this nomogram was developed from a data set of patients who had undergone R0 resections without any adjuvant therapy. The concordance index of the nomogram was 0.64 for patients who received postoperative chemoradiation, which was lower than the concordance index for patients who received no adjuvant therapy (0.80). The observed survival of patients receiving postoperative chemoradiation was approximately 20 % higher than that predicted by the nomogram. The authors concluded that the MSKCC nomogram significantly underpredicted the survival of this patient group, confirming the survival benefit conferred by postoperative chemoradiation. This study highlights the need to update current postoperative nomograms with incorporation of patient cohorts receiving adjuvant therapy.
3.1.2 Preoperative Chemoradiotherapy
Several investigators have reported prognostic factors for survival after preoperative chemoradiotherapy for gastric cancer. In the RTOG 99-04 phase II study of preoperative chemoradiotherapy, Ajani et al. reported that 82 % of patients with a pCR following preoperative chemoradiotherapy were alive compared with 69 % of patients with less than pCR (Ajani et al. 2006). A similar correlation between pCR and overall survival in gastric cancer patients treated with preoperative chemoradiation has been reported by other groups (Ajani et al. 2004; Reed et al. 2008). Rostom et al. reported a study of 41 patients with operable gastric cancer who were treated with 2 cycles of induction chemotherapy (fluorouracil, docetaxel, and cisplatin) followed by 45 Gy of radiation with concurrent fluorouracil and docetaxel, and then surgery (Rostom et al. 2012). The pCR rate was 24 % and the 3 year overall survival rate was 47.3 %. In this study, overall survival was significantly correlated with pathological response, R0 resection, dissected pathologically positive lymph nodes, and post-surgery T-stage. Investigators from the MD Anderson Cancer Center investigated the role of surgical pathology stage following preoperative chemoradiation as a prognosticator of overall survival (Rohatgi et al. 2006). The patient cohort for this study comprised 74 patients enrolled on 2 prospectively conducted, preoperative chemoradiation trials. All patients were staged with endoscopic ultrasound (EUS) and laparoscopy and were treated with induction chemotherapy followed by chemoradiation to 45 Gy, and then surgery. This analysis did not show any correlation between baseline clinical stage as determined by EUS and overall survival. However, there was a significant correlation between postsurgical T, N, and overall stages with overall survival. Of all the factors assessed, the pathologic AJCC stage was the strongest predictor of overall survival. Patients with stages 0-II tumours survived significantly longer than patients with stages III-IV tumours (median time not reached vs 12 months respectively; p = 0.001). In addition, patients who achieved a pCR survived significantly longer than those who did not (median time not reached versus 53 months; p = 0.004). The authors concluded that when preoperative chemoradiation is employed, the surgical pathology stage is a better prognosticator of overall survival than the baseline clinical stage. In a separate study utilising the same patient cohort, the same investigators have shown that clinical stage after preoperative chemoradiation is also a better predictor of patient outcome than baseline clinical stage (Patel et al. 2007). Amongst the 74 patients who received preoperative chemoradiation, 35 underwent repeat preoperative staging with EUS. For these patients, the correlation between baseline EUS stage and overall survival was not statistically significant. However, there was a significant correlation between pre-surgical EUS stage and overall survival. Patients with pre-surgical stages I-II tumours survived significantly longer than patients with pre-surgical stages III-IV tumours (median time not reached versus 15.2 months respectively; p = 0.01).
3.2 Molecular Biomarkers
Although there are many reports describing molecular biomarkers associated with response to chemotherapy in gastric cancer (Mutze et al. 2011; Robb and Mariette 2012; Van Cutsem et al. 2012; Wang et al. 2012), there is very little published data relating to molecular biomarkers associated with the use of chemoradiotherapy. This reflects the relatively short period of time that radiotherapy has been used as part of curative treatment for gastric cancer. Lee et al. investigated the impact of E2F-1 expression on the clinical outcome of gastric cancer patients treated with surgery and adjuvant chemoradiation (Lee et al. 2008). E2F-1 is a transcription factor that can act either as an oncogene or a tumour suppressor gene depending on the primary tumour type, and it controls the transcription of several genes involved in DNA synthesis (Bell and Ryan 2004; La Thangue 2003; Wu et al. 2001). The patient cohort for this study comprised 467 patients from a single centre in Korea who all underwent D2 gastrectomy followed by adjuvant chemoradiation using the INT0116 regimen (45 Gy of radiation plus 5-FU and leucovorin). Immunohistochemical studies using tissue microarrays showed that E2F-1 immunopositivity predicted more favourable survival as compared with E2F-1 immunonegativity [p = 0.050, hazard ratio = 0.702, 95 % confidence interval 0.487–1.013]. However, AJCC stage was still the most powerful prognostic factor in this series. Soyuer et al. investigated the prognostic significance of CD9 expression in 49 patients with locally advanced gastric cancer treated with surgery and adjuvant chemoradiation (Soyuer et al. 2010). CD9 is a tetraspanin transmembrane protein that plays an important role in inhibiting cell motility in several tumour cell lines, including gastric cancer. Immunohistochemical evaluation showed CD9 positivity to be a significant prognostic factor for disease-free and overall survival. Ongoing and future clinical trials will continue the search to identify molecular biomarkers of response and survival, but currently there are no reliable prognostic markers to identify gastric cancer patients who may benefit from adjuvant chemoradiotherapy.
4 Toxicity of Chemoradiotherapy for Gastric Cancer
4.1 Acute and Late Toxicities
A major concern amongst radiation oncologists when contemplating gastric irradiation is toxicity. Radiotherapy to the abdomen can result in significant acute toxicity related to hepatic and gastrointestinal mucosal exposure. In addition, gastric irradiation may also affect critical normal tissues such as the kidneys, liver, lungs, and heart, resulting in late toxicities. The combined modality regimen employed in INT0116 was associated with considerable acute toxicity, with grade 3 and grade 4 toxicity occurring in 41 and 32 % of cases, respectively (Macdonald et al. 2001). Only 64 % of patients completed treatment as planned and 17 % of patients were unable to complete radiotherapy due to treatment related toxicity. The main toxicities associated with this treatment were haematologic and gastrointestinal. Major grade 3/4 haematologic toxicity occurred in 54 % of patients while major grade 3/4 gastrointestinal toxicity occurred in 33 % of patients. There have been no reports of late treatment-related toxicities with long-term follow-up, although 21 patients in the chemoradiation arm developed second malignancies compared to 8 in the observation arm (Smalley et al. 2012). Other investigators have also reported high toxicity rates when using the INT0116 regimen. In the study by Chang et al. of 120 patients treated with the INT0116 regimen, the reported rate of grade 3 or greater acute toxicity was 66 %, while the rate of grade 4 toxicity was 22 % (all due to neutropenia) (Chang et al. 2011). Grade 3 gastrointestinal toxicity occurred in 8 % of patients while grade 3/4 haematologic toxicity occurred in 61 % of patients. The authors reported that anaemia and gastritis were the most commonly occurring late complications of treatment. Twelve patients developed gastritis that was confirmed by gastroscopy and was either grade 1 or 2. Three patients developed anastomotic strictures, 2 patients developed malabsorption, and 1 patient developed a bowel obstruction. Grade 1 renal impairment was observed in 3 patients and was manifested clinically as mildly elevated serum creatinine or proteinuria. Interestingly, ultrasound and CT performed on these 3 patients demonstrated atrophic left kidneys that were attributed to radiation. Kundel et al. have recently evaluated the tolerability of the INT0116 regimen in 166 patients treated over a 7 year period (Kundel et al. 2011). Treatment compliance was relatively poor with only 54 % of patients completing the entire chemoradiation regimen (87 % completed radiotherapy and 57 % completed chemotherapy). In all cases, treatment discontinuation was due to treatment related toxicity. Acute toxicity was considerable with 46 % of patients experiencing grade ≥3 toxicity. Grade ≥3 haematologic toxicity occurred in 32 % of patients with the most common being neutropenia and leukopenia (grade ≥3 in 30 and 25 % respectively). Febrile neutropenia was reported in 15 % of patients. Grade ≥3 non-haematologic toxicity occurred in 25 % of patients with the most common being nausea, vomiting and diarrhoea (10 % for each toxicity). Three patients (1.8 %) died from treatment related toxicity and 48 patients (29 %) were hospitalised for toxicity. Late radiation toxicity was not reported for this study.
It should be noted that the majority of patients in the above studies were treated according to the original INT0116 protocol i.e., bolus 5-FU/leucovorin chemotherapy combined with radiotherapy delivered using AP-PA fields. In the decade that has elapsed since publication of the INT0116 trial, several alternative chemoradiation regimens for gastric cancer have been reported that combine more current systemic treatment with modern techniques of radiotherapy delivery. The aim of any new regimen is to enhance the therapeutic ratio by improving efficacy and reducing toxicity. We recently reported the results of a prospective, multi-centre study that evaluated a regimen of ECF chemotherapy combined with chemoradiation (Leong et al. 2011). In this study, 54 patients received adjuvant treatment consisting of 1 cycle of ECF chemotherapy, followed by radiotherapy (45 Gy) with concurrent continuous infusional 5-FU, and then 2 further cycles of ECF. All patients were treated using multiple-field 3D conformal techniques (Leong et al. 2005




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree