Fig. 1
7th edition AJCC breast cancer staging. AJCC staging reprinted with kind permission, Springer Publications
3 Prognostic Factors in Breast Cancer
3.1 Clinical Factors
Until recently, clinical factors have largely guided treatment decision-making and discussions about prognosis with individual patients. Factors that can increase the risk for breast cancer include female gender, family history of breast cancer, genetic mutations, particularly BRCA1 and BRCA 2 mutations, and increasing age. Increasing exposure to estrogens, including early menarche, late menopause, nulliparity or older age at first childbirth, and exogenous hormone replacement therapy are all associated with an increasing risk of breast cancer (Carlson et al. 2013). Lobular carcinoma in situ is a risk factor for development of invasive breast cancer in either breast of greater than 20 % at 15 years (Haagensen et al. 1981). Pleomorphic lobular carcinoma in situ has been reported as a more aggressive variant with higher propensity to develop into invasive breast cancer, and these are typically managed with surgical excision when found on biopsy (Anderson et al. 2006). Age is one of the most important clinical factors, as numerous reports have shown that younger patients have a higher risk of breast cancer recurrence (Zhou et al. 2004). On recursive partitioning analysis of prognostic factors for breast cancer outcomes, age is the first split in the tree (Freedman et al. 2002). Location of the tumor within the breast does not affect outcomes, though it can have an effect on cosmesis (Freedman et al. 2002).
Axillary nodal status on clinical staging portends a worse clinical outcome (Carter et al. 1989). The staging described earlier divides pathological disease in the axilla into numerous categories, with published papers showing a worse outcome for node positive patients (Dent 1996) as well as greater nodal burden (Cabanes et al. 1992) versus nodal negative patients (Jatoi et al. 1999). Axillary surgical staging is recognized as a diagnostic and therapeutic procedure (Moore and Kinne 1997). While this was debated for some time as to whether it showed a lead time bias in catching node positive cancers later in their natural history, it is now clear that these tumors are biologically different than node negative cancers (Mittra 1993).
Clinical stage is correlated with outcome, with higher tumor and nodal stage having worse clinical outcomes. Inflammatory breast cancer, described in the staging as T4d, shows a poor outcome regardless of other prognostic factors at the time of diagnosis (Chang et al. 1998). With the poorer outcomes for inflammatory breast cancer based on clinical factors, management involves tri-modality therapy consisting of radiation therapy with dose escalation to decrease recurrence (Liao et al. 2000), cytotoxic chemotherapy, and mastectomy with axillary nodal dissection in all cases where feasible (Cristofanilli et al. 2003).
3.2 Pathologic Factors
The pathology report after breast-conserving surgery or mastectomy remains a crucial driver of adjuvant treatment and outcomes. The effect of margin status remains hotly debated after decades of papers and discussions in the breast cancer literature. Some define a negative margin as no tumor at ink, and this has been the standard in all National Surgical Adjuvant Breast and Bowel Program (NSABP) trials that have driven clinical practice (Wapnir et al. 2011). Tumor at ink has been shown to correlate with recurrence rates (Macmillan et al. 1997). Others chose 2 mm as the definition of a negative margin, and papers published show this associated with a very low risk of ipsilateral breast tumor recurrence after breast conservation, while those with a smaller margin are at a similar risk to those with tumor at ink (Freedman et al. 1999). More recent papers, taking into account better biologic understanding and systemic therapy of tumors, conclude that no tumor at ink is a sufficient negative margin in most clinical scenarios, similar to the NSABP practice (Morrow et al. 2012).
Margin status has also been investigated closely in ductal carcinoma in situ, with wider margins associated with fewer recurrences in some studies (MacDonald et al. 2005). Grade has proven to be an important factor in the recurrence of ductal carcinoma in situ. For high grade patients who have lumpectomy without radiation, the observed recurrence rate is 15.3 % at 5 years, or over 3 % per year. In patients with low to intermediate grade ductal carcinoma in situ, the rate is closer to 1 % per year (Hughes et al. 2009).
Tumor markers are important in breast cancer prognosis. Estrogen receptor status, including the amount of estrogen receptor positivity and the percentage of cells staining positive, should be reported on the pathology report after breast conservation surgery and mastectomy. Estrogen receptor quantity is prognostic for survival from breast cancer (Shek et al. 1989). Estrogen receptor status by immunohistochemistry is superior to ligand binding (Harvey et al. 1999). Estrogen receptor status is linked to long term survival prognosis (Crowe et al. 1991) and is an established prognostic factor in breast cancer (Speirs and Kerin 2008). Progesterone receptor status will be similarly reported, and this usually but not always mimics estrogen receptor expression. Progesterone is also a clinical prognostic factor (Liu et al. 2010).
HER2/neu oncogene amplification is a recognized prognostic factor in both recurrence and survival (Slamon et al. 1987). This oncogene is a member of the Epidermal Growth Factor Receptor (EGFR) family (Yamamoto et al. 1986) and retains significant prognostic power after multivariate analysis. This family is known to play an important role in cancer progression (Holbro et al. 2003). Node positive patients have inferior survival with HER2/neu oncogene amplification (Borg et al. 1990). In multivariate analysis, gene amplification was the second most important prognostic factor after axillary nodal status (Paik et al. 1990).
Nodal staging has undergone significant changes in the AJCC staging system (American Joint Committee on Cancer 2002). One of the major areas of debate is how to handle cells that are found on immunohistochemistry or molecular testing and whether this merits full axillary dissection (Teng et al. 2000). They have now been found to behave more similarly to node negative patients (Cote et al. 1999) and the updated breast staging reflects this by the designation as N0(i +) or N0(mol +) (American Joint Committee on Cancer 2009). Pathologic tumor size and nodal status remain significant predictors of locoregional recurrence after mastectomy (Buchholz et al. 2002).
Triple negative breast cancers are a less common subtype that are negative for estrogen receptors, progesterone receptors, and HER2/neu oncogene amplification. These tumors have a worse prognosis than tumors with tumor marker positivity. Prognostic factors in these triple negative tumors include lymph node status and tumor size, while other parameters that did not reach significance on multivariate analysis include age, histological grade, tumor size, and vascular invasion (Rakha et al. 2007). Triple negative breast cancers have been shown to have elevated intratumoral levels of Vascular Endothelial Growth Factor (VEGF) expression which is correlated with poorer survival (Linderholm et al. 2009).
Recurrent breast cancer represents an unfavorable subset (Lee et al. 2006), yet prognostic factors can be identified within this group to help differentiate outcomes (Pater et al. 1981). Estrogen and progesterone status play an important role in survival from recurrent breast cancer (Howat et al. 1985). Nodal status also plays an important role in survival from recurrent breast cancer (Shek et al. 1987).
3.3 Adjuvant! Online
Numerous prognostic factors have been identified and published in the breast cancer literature. The most robust non-genomic prognostic tool to incorporate this vast literature is Adjuvant! Online, an online tool that allows oncologists to determine quantitative data for their patients in order to make medical decision-making, based initially off of the SEER database (Ravdin et al. 2001). This has been separately validated in the British Columbia Tumor Registry (Olivotto et al. 2005) and is in frequent clinical use and cited in the NCCN guidelines for management of breast cancer (Carlson et al. 2013).
Strengths of Adjuvant! Online is its ease of use and accessibility to clinicians anywhere with internet access. It can be easily opened at consultations and help reassure the patient about outcomes and guide the conversation. One major limitation is the lack of incorporation of HER2 status, which is a major prognostic factor, into its model. For radiation oncologists, it cannot be used as a predictive tool to look at survival with and without radiation, as can some of the nomograms presented later in this chapter.
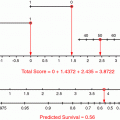
An example is illustrated for a 65 year old woman with minor medical comorbidities with a pT1cN0 ER + Grade 1 breast cancer. Looking at the 10 year risk for mortality, one calculates a 84.7 % chance of the patient being alive in 10 years, with a 2.8 % risk of dying of disease and 12.5 % risk of dying of other causes. The data was based off of mortality data, and this remains the most robust use of this tool, though it can also be used to look at recurrence.
3.4 Oncotype DX
The Oncotype DX is a 21 gene assay that produces a recurrence score for women based on 16 cancer genes and five reference genes (Paik et al. 2004). The ability to use paraffin embedded tissue to extract these 21 genes was separately validated (Esteva et al. 2005). The assay was developed for use in estrogen receptor positive, node negative invasive, non-metastatic breast cancer and validated in numerous populations including SWOG 8814 (Albain et al. 2010).
The use of the Oncotype DX has subsequently expanded into different populations. It has now been validated in the node positive population, allowing novel approaches to this group who have traditionally all been offered chemotherapy (Dowsett et al. 2010).
Advantages of the Oncotype DX are that one can use paraffin-embedded tissue, which is easier to attain or send post-operatively than fresh tissues. It provides a more individualized picture of each patient’s recurrence score than the Adjuvant! Online, and does incorporate HER2 overexpression as one of the genes investigated, unlike Adjuvant! Online. Prior to the advent of the Oncotype DX assay, chemotherapy for node negative, estrogen receptor positive women was commonly given to those with a tumor size of 1 cm or greater, whereas now many of these women are spared chemotherapy that will have little to no benefit in low Oncotype DX recurrence score patients.
Disadvantages include that many patients fall into an intermediate score category, where the predictive value for chemotherapy benefit is less certain than a low score where anti-estrogen therapy alone is likely sufficient or a high score where the margin of benefit of chemotherapy is much greater. The Trial Assessing IndividuaLized Options for Treatment (TAILORx) investigated this population with intermediate Oncotype DX scores, randomizing individuals to either anti-endocrine therapy alone or with the addition of chemotherapy but results are not yet available (Zujewski and Kamin 2008).
Oncotype DX has also been shown to predict for locoregional recurrence when used on patients from the NSABP trials B-14 and B-20. In particular, the mastectomy patients treated without radiation had excellent predictive value, suggesting that adjuvant radiation therapy could potentially be tailored to individual patient risk in this group (Mamounas et al. 2010).
Oncotype DX for ductal carcinoma in situ is also available to help guide recurrence risk in this population. Similarly to the Oncotype DX for invasive breast cancer, a tissue specimen is used to look at 21 genes and predict a local recurrence score from 0 to 100 for ductal carcinoma in situ or an invasive recurrence based on the results. The Oncotype DX for ductal carcinoma in situ was validated in a population of women from the ECOG E5194 trial (Hughes et al. 2009), presented in 2011 (Solin et al. 2011), and is now clinically available to help guide decision making. The low score (0–38) predicts a 12 % risk of local recurrence in the next 10 years, an intermediate score (39–54) predicts a 24.5 % recurrence rate, and a high score (55 or higher) predicts a 27.3 % risk of a local recurrence in 10 years. From the validation group in ECOG E5194, 75 % of women ended up in the low risk group (Solin et al. 2011). This test is useful to help quantify the risk of recurrence and potentially guide treatment decisions (Fig. 2).


Fig. 2
Oncotype report for node negative breast cancer. Oncotype report reprinted with kind permission, genomic health 2013
3.5 Mammaprint as a Prognostic Tool
Mammaprint has subsequently been introduced as a 70 gene assay and validated to have prognostic value in overall survival (van der Vijver et al. 2002). Multivariate analyses show that even when controlling for other clinical factors, the assay remains a strong predictor of overall survival. One major limitation of the Mammaprint in the United States has been the need for fresh tissue, rather than the ability to use paraffin-embedded tissue as with the Oncotype DX. A newer version that can use paraffin-embedded tissue, Symphony, has been introduced and is currently incorporated into ongoing clinical studies as a prognostic tool. An example of a mammaprint result is shown below (Fig. 3).
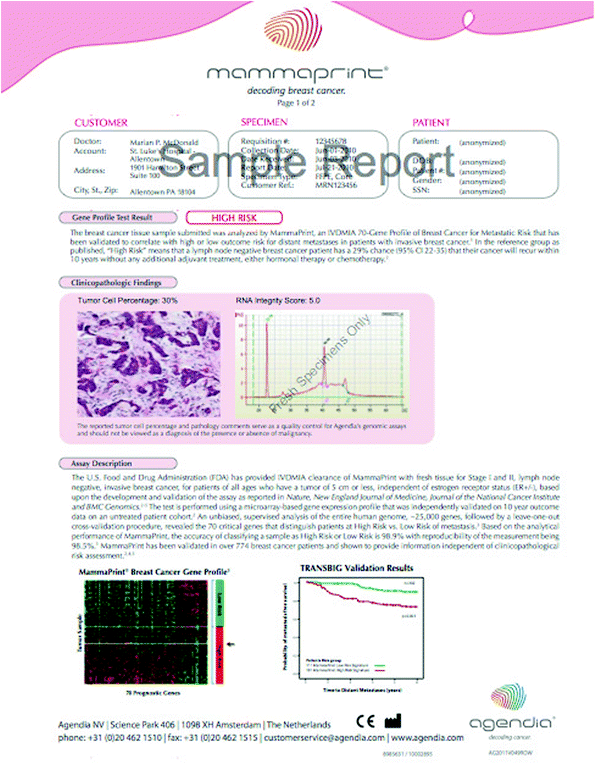

Fig. 3
Mammaprint report for a high risk patient. Mammaprint report provided with kind permission, Agendia 2013
4 Predictive Factors and Tools in Breast Cancer
4.1 Clinical Factors
Imaging has proven useful to determine stage as well as potential treatment options. Magnetic resonance imaging (MRI) has been extensively studied, and has been shown to alter the surgical options in up to one-third of women who undergo MRI of the breast (Houssami et al. 2008). However, there is no prospective trial data showing a difference in clinical outcomes when MRI is used and retrospective studies do no suggest a difference (Solin et al. 2008).
Radiation has been demonstrated to change the failure pattern when used in the post-mastectomy setting, decreasing locoregional failures including chest wall (Nielsen et al. 2006).
Women who are staged as pathologic N2 (4 or more lymph nodes) or higher will have an overall survival benefit from post-mastectomy radiation. There is some suggestion that the survival benefit is greatest in those with the appropriate biological equivalent dose of 40–60 Gy in 2 Gy fractions and appropriate controls and targeting for radiation delivery (Gebski et al. 2006).
4.2 Pathologic Factors
All breast cancer pathology reports should include estrogen and progesterone receptor status and quantification as well as HER2 overexpression. These three markers remain the most important in guiding choice of adjuvant systemic therapy, as well as being important prognostic tools. They are all incorporated into genomic tools including Oncotype DX and Mammaprint. Patients with estrogen responsive tumors should benefit from anti-endocrine therapy as their final adjuvant treatment for at least 5 years, though some data suggests that longer treatment may have further benefit. The choice of treatment for premenopausal women is tamoxifen, and for women without functional ovaries, aromatase inhibitors are first line therapy. Both of these typically follow radiation therapy.
For women with HER2 overexpression of 3+ by immunohistochemistry or on fluorescence in situ hybridization (FISH) testing, trastuzumab is typically used in addition to cytotoxic chemotherapy (Romond et al. 2005). Until recently, women that had some degree of overexpression by immunohistochemistry (1+ −2+) did not realize any predictive value from this as trastuzumab is used only in HER2 3+ overexpression. However, these tumors that are node positive and 1+ −2+ are now eligible for Neuvax, a peptide vaccine targeting the HER2 protein that has demonstrated efficacy in women with overexpression (Peoples et al. 2005).
Axillary nodal status remains the top pathologic factor that drives adjuvant treatment choices. In clinically node negative patients, the standard procedure to accompany breast surgery is sentinel lymph node biopsy, as long as technically successful. Much of the data for predicting nodal positivity is based on axillary dissections prior to the sentinel lymph node trial results.
4.3 Introduction to Nomograms in Breast Cancer Management
Nomograms have been developed to predict risks of recurrence and help guide treatment in numerous breast cancer scenarios. They include estimation risk of sentinel and non-sentinel lymph node positivity to help guide axillary dissection, risk of recurrence from DCIS, early breast cancer, advanced breast cancer, and even metastatic disease. There are also powerful predictive tools to help guide systemic therapy based on individual tumor genetic markers that have rapidly changed the breast cancer landscape. We will look at management decisions for in situ carcinomas, early invasive carcinomas, advanced carcinomas, and metastatic disease separately. Many predictive tools to help guide treatment decisions and outcome discussions have been developed in breast cancer.
Different predictive tools have been developed and broadly classified into nomograms. Kattan defined a nomogram more narrowly as a graphical calculation instrument that can be based on any type of function, such as logistic regression or Cox hazard ratio regression models (Kattan 2001).
4.4 Nomograms for Predicting Outcomes and Guiding Treatment in Ductal Carcinoma In situ
Breast cancer cells that are lining the ductal system, commonly causing signature calcifications, but have not invaded the basement membrane are known as ductal carcinomas in situ (DCIS) (Rudloff 2010).
Memorial Sloan Kettering physicians developed a DCIS recurrence nomogram that quantifies, based on known risks factors, the risk of a DCIS recurrence. Factors that are included in this nomogram are listed in (Table 1).
Table 1
DCIS nomogram factors
Age at diagnosis (25–90) | Family history of breast cancer |
Presentation (physical exam or imaging) | Year of surgery |
Use of adjuvant radiation therapy | Use of adjuvant endocrine therapy |
Nuclear grade | Association of necrosis with DCIS |
Surgical margins | Number of surgical excisions |
Since this nomogram includes potential adjuvant treatments, one can quantify five and 10 year recurrence rates for patients with different possible treatment scenarios. For instance, a 50 year old woman with one surgery showing negative margins, no family history, and intermediate grade DCIS with a radiologic presentation in 2012 can have her risk of recurrence quantified with and without radiation therapy and with and without endocrine therapy. This flexibility is very helpful for clinicians trying to individualize care for each patient.
4.5 Nomograms for Predicting Sentinel Lymph Node Involvement
Large randomized studies have been done investigating the equivalence of sentinel node biopsy to axillary lymph node dissection (Veronesi et al. 2003). Initial concerns over the sentinel node procedure included the fact that the recurrence rate might be higher than with axillary nodal dissection, but this is not the case in large series (Naik et al. 2004).
Predictive factors for likelihood of axillary nodal involvement have been published and are incorporated into nomograms predicting likelihood of sentinel node involvement (Gann et al. 1999). This has also been reported in early breast cancer patients, limited to T1a and T1b tumors to help determine who might have the highest likelihood of axillary nodal metastases (Rivadeneira et al. 2000). Large series have also been published looking at what factors women with axillary nodal metastases and early breast tumors have in common (Maibenco et al. 1999). Histopathology has been shown to play a role in the likelihood of early breast tumors with positive lymph nodes (Mustafa et al. 1997).
The likelihood of an involved sentinel lymph node has become an important predictor for prognosis with implications for systemic therapy and its sequencing, particularly with consideration of neoadjuvant systemic therapy. Neoadjuvant cytotoxic therapy has been used in randomized clinical trials to allow some additional patients to undergo breast conservation therapy rather than mastectomy, and ongoing trials are evaluating the role of neoadjuvant hormonal therapy. Many clinicians are not obtaining a pre-treatment sentinel node in a patient with a clinically negative axilla, so predictive tools play a crucial role in this population (Jackson et al. 2000).
Bevilacqua and colleagues have developed a tool that can predict the probability of tumor spread to sentinel lymph nodes. The factors utilized in this tool are listed in (Table 2) (Bevilacqua et al. 2007).
Table 2
Nomogram for sentinel node involvement
Patient age | Tumor size |
Tubular, colloid/mucinous, or medullary | Location in upper inner quadrant only |
LVSI | Multifocality |
Tumor type (lobular vs. ductal) | If ductal, histologic grade (I–III) |
Estrogen receptor status | Progesterone receptor status |
Strengths of this nomogram include the fact that all data can be used pre-operatively. The model was developed based on a cohort of 3,786 women and validated on a separate cohort of 1,545 women with sentinel node biopsies. This yielded an area under the receiver operating curve of 0.754. This information is very useful to radiation oncologists and surgeons in deciding how to assess the nodal risk of tumor involvement and make decisions regarding local therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree