Score
Bilirubin (mg/dL)
Albumin (g/dL)
Prothrombin time (sec)
Hepatic Encephalopathy (grade)
Ascites
1
<2
>3.5
<4
None
None
2
2–3
2.8–3.5
4–6
1–2
Mild (detectable)
3
>3
<2.8
>6
3–4
Severe (tense)
Child class: A, 5–6; B, 7–9; C, > 9
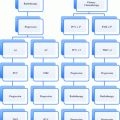
Commonly used staging systems for hepatocellular carcinoma include the American Joint Committee on Cancer (AJCC) TNM staging system (Table 2), as well as the Barcelona Clinic Liver Cancer (BCLC) classification (Fig. 1). A criticism of the BCLC is that patients with portal invasion, node positive, and/or metastasis positive disease are collectively grouped, and focal therapies are not recommended for this heterogeneous cohort of patients. Other staging systems including the Okuda staging system (Okuda and Obata et al. 1985) and Cancer of the Liver Italian Program (CLIP) scoring system (Kanematsu and Hoshi et al. 1999), which are clinical staging systems best suited for patients with poor liver reserve and advanced disease. The presence of portal vein thrombosis alone has been shown to portend a poor prognosis, with a median survival of 3 months without treatment for these patients (Wang and Zhang et al. 2008).
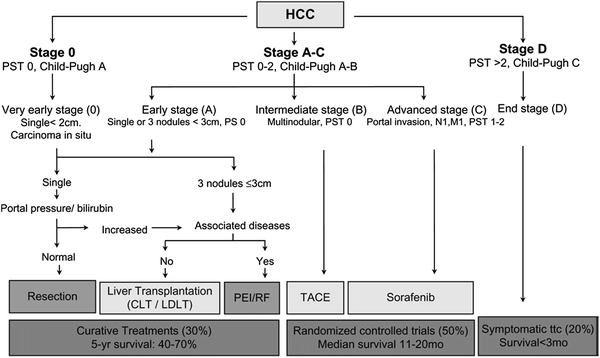
Table 2
TNM Classification for Liver Tumors from the American Joint Committee on Cancer (AJCC) 7th edition (2010)
Primary Tumor (T) | |||
TX | Primary tumor cannot be assessed | ||
T0 | No evidence of primary tumor | ||
T1 | Solitary tumor without vascular invasion | ||
T2 | Solitary tumor with vascular invasion or multiple tumors none more than 5 cm | ||
T3a | Multiple tumors more than 5 cm | ||
T3b | Single tumor or multiple tumors of any size involving a major branch of the portal vein or hepatic vein | ||
T4 | Tumor(s) with direct invasion of adjacent organs other than the gallbladder or with perforation of visceral peritoneum | ||
Regional Lymph Nodes (N) | |||
NX | Regional lymph nodes cannot be assessed | ||
N0 | No regional lymph node metastasis | ||
N1 | Regional lymph node metastasis | ||
Distance Metastasis (M) | |||
M0 | No distant metastasis | ||
M1 | Distant metastasis | ||
Anatomic stage/Prognostic groups | |||
Stage I | T1 | N0 | M0 |
Stage II | T2 | N0 | M0 |
Stage IIIA | T3a | N0 | M0 |
Stage IIIB | T3b | N0 | M0 |
Stage IIIC | T4 | N0 | M0 |
Stage IVA | Any T | N1 | M0 |
Stage IVB | Any T | Any N | M1 |

Fig. 1
Barcelona Clinic Liver Cancer (BCLC) classification for hepatocellular carcinoma (HCC). Llovet and Di Bisceglie et al. (2008) Barcelona Clinic Liver Cancer (BCLC) classification for hepatocellular carcinoma (HCC) including incidence, estimated survival, and management options. PST, performance status based on Eastern Cooperative Oncology Group score; N nodal stage; M metastases stage; CLD cadaver liver transplantation; LDLT living donor liver transplantation; RF radiofrequency ablation; PEI percutaneous ethanol injection; TACE transarterial chemoembolization (Llovet and Di Bisceglie et al. 2008)
2.2 Treatment-Specific Factors
The majority of patients with hepatocellular carcinoma are asymptomatic, and symptomatology from primary liver cancer portends a poor prognosis. Treatment options for hepatocellular carcinoma are dependent upon underlying liver function. Less than 30 % of patients present with potentially resectable, and thereby potentially curably disease (Bruix and Sherman 2005). Five-year survival for this favorable cohort of patients ranges between 40 and 70 %. Approximately, 20 % of patients are diagnosed with end stage, symptomatic disease. Treatment strategies for these patients are palliative in nature, and median survival is less than 3 months. The remainder of patients, approximately 50 %, are diagnosed with inoperable, locally advanced disease (Llovet and Bruix et al. 2003), and therapeutic modalities may include chemotherapy, percutaneous ablation, embolization, conformal radiotherapy, and/or SBRT.
2.2.1 Liver Transplantation in the Management of Hepatocellular Carcinoma
Liver transplantation is the gold standard treatment for hepatocellular carcinoma, as both the cirrhotic liver and primary disease are eradicated. The United Network for Organ Sharing (UNOS) uses the Milan criteria to identify patients who are suitable for transplant, which stipulates that one tumor should be ≤5 cm, or up to 3 tumors could measure up to 3 cm each, without extrahepatic spread or macrovascular invasion. For patients with these favorable features, the 4-year survival rate was reported to be 85 %, and the recurrence-free survival was 92 % (Mazzaferro and Doci et al. 1996).
Liver transplantation is limited by organ availability, and approximately 20 % of patients who have been listed on the liver transplant list have subsequently withdrawn due to tumor progression. Therefore, an interest in “bridge” therapies has emerged to maintain patient eligibility for liver transplant. Traditional bridge therapies have included radiofrequency ablation (Lu et al. 2005), chemoembolization (Heckman and Devera et al. 2008), and radioembolization with yttrium-90 microspheres (Kulik and van Holsbeeck et al. 2006). Hepatic resection as a bridge to transplant yielded higher operative mortality, increased recurrence, and poorer outcome than patients receiving primary liver transplantation in a prospective, single institution French experience, and has subsequently been abandoned as a bridge therapy (Adam and Azoulay et al. 2003).
More recently, SBRT has emerged as a viable bridge to transplant strategy. The Baylor experience reported a 5-year overall survival rate of 100 % for 10 patients with hepatocellular carcinoma who received bridge therapy in the form of SBRT to a median dose of 51 Gy in 3 Gy fractions prior to orthotopic transplant (O’Connor and Davis et al. 2012). The University of Rochester experience, which treated 11 evaluable lesions in 10 patients to a median dose of 50 Gy in 5-Gy fractions, reported no grade ≥ 3 toxicities and no radiation-induced liver disease. All patients were alive at a median follow-up of 19.6 months (Katz and Chawla et al. 2012). Finally, a prospective phase I-II trial conducted at indiana university treated 14 hepatocellular carcinoma patients with a Child-Pugh Class B score of <8 were treated to a total dose of 40 Gy in 5 fractions. Local control at 12 months was 87.5 % (Cardenes and Lasley et al. 2012). Three grade 4 toxicities were observed, which included hyperbilirubinemia, hypokalemia, and thrombocytopenia.
2.2.2 Surgical Resection in the Management of Hepatocellular Carcinoma
Hepatic resection is a potentially curative option for patients with early stage disease. Prediction of post-operative hepatic reserve is an essential component of the preoperative evaluation of these patients. To assess for the adequacy of the future liver remnant, the Child-Pugh classification has historically been employed (Table 1). In North America, the current evaluation for resection typically includes the determination of the presence of portal hypertension, as quantified by hepatic vein catheterization (Bruix and Castells et al. 1996). Other methods to clinically assess for portal hypertension include the presence of splenomegaly and/or thrombocytosis. The literature is mixed regarding the exact margin required for adequate removal of the tumor, although 1–2 cm is generally recommended.
2.2.3 Percutaneous Ablation in the Management of Hepatocellular Carcinoma
Percutaneous ablation is a local therapy whereby substances (alcohol or acetic acid) are injected into a focal liver lesion, or the temperature is changed (via radiofrequency ablation, microwave, laser, or cryotherapy). Ethanol and radiofrequency ablation are the two most commonly reported ablative techniques. In a retrospective analysis of five randomized trials, it appears that radiofrequency was superior to ethanol ablation in terms of overall survival and local control for small HCC (Chen and Li et al. 2006). Radiofrequency ablation was directly compared to hepatic resection in a randomized trial of 230 patients conducted in China. Hepatic resection was found to be significantly superior to radiofrequency ablation, with an overall survival rate of 75.6 % versus 54.8 %, and recurrence-free survival of 51.3 % versus 28.7 %, respectively (Huang and Yan et al. 2010). The NCCN currently endorses ablation as a local treatment modality for patients with a small burden (tumors ≤ 3 cm) of unresectable disease (Benson and Abrams et al. 2009). Lesions not suitable to ablation include lesions near the dome, as they are less well visualized with ultrasound, and lesions near large blood vessels, due to concerns of heat sink from nearby circulation.
2.2.4 Endovascular Therapies in the Management of Hepatocellular Carcinoma
Endovascular therapies for the treatment of hepatocellular carcinoma include radioembolization and chemoembolization. These techniques exploit the differential perfusion of hepatic malignancies and parenchyma, given that tumor cells derive their blood supply from the hepatic artery, whereas normal hepatocytes rely on the hepatic vein. In this way, cancerous cells are preferentially targeted with relative sparing of normal hepatocytes. Radioembolization is a catheter-based, liver-directed therapy that involves the hepatic intra-arterial injection of isotopes, typically yttrium-90, bound to resin microspheres. While the major mechanism of radioembolization is due to radiation, embolization also attempts to induce ischemic necrosis of the tumor. In contrast, transcatheter arterial chemoembolization attempts to both increase the local concentration of chemotherapeutic agents and induce ischemic necrosis of the tumor. In hepatocellular carcinoma, chemoembolization and radioembolization have been used to delay disease progression, bridge to potential liver transplantation, or palliate symptoms.
Multiple randomized trials comparing transcatheter arterial chemoembolization versus best supportive care for patients with unresectable hepatocellular carcinoma and without severe liver disease found no advantage to transarterial chemoembolization versus conservative therapy (Pelletier 1990; Hepatocellulaire 1995), although a meta-analysis of 7 randomized, controlled trials found a significant 2-year overall survival benefit of chemoembolization versus control (Adam and Azoulay et al. 2003). The NCCN endorses chemoembolization as a treatment option for patients with unresectable hepatocellular carcinoma without main portal vein thrombosis, as ischemic hepatitis is a known complication of this procedure (Benson and Abrams et al. 2009).
A recent comparative analysis study of patients with unresectable hepatocellular carcinoma without portal vein thrombosis compared 122 patients who received chemoembolization and 123 who received radioembolization (Salem and Lewandowski et al. 2011). The acute side effects of abdominal pain and transaminitis were significantly more frequent in the chemoembolization cohort. Time to progression was statistically improved from 8.4 to 13.3 months, favoring patients who underwent radioembolization as opposed to chemoembolization. Various older, retrospective studies have concluded that radioembolization and chemoembolization are equivalent locoregional therapies with similar effectiveness and safety profiles (Poon and Lau et al. 2007; Shannon and Williams 2008).
The most commonly used radioisotope for radioembolization is yttrium-90 (90Y), which is a pure beta-emitter with a half-life of 64.2 h. In a recent, prospective report of long-term outcomes of patients receiving 90Y, overall time to progression was 7.9 months (Poon and Lau et al. 2007). Patients with Child-Pugh A disease, with or without portal vein thrombosis, enjoyed the longest survival. Survival times significantly differed between patients with Child-Pugh A and B disease, at 17.2 months and 8.8 months, respectively. Patients with Child-Pugh B disease who had portal vein thrombosis fared the poorest, with a median survival of 5.6 months. Baseline patient characteristics, including age and performance status, as well as serum indicators of hepatic function predicted for survival.
2.2.5 External Beam Radiotherapy in the Management of Hepatocellular Carcinoma
Prior to the development of conformal radiotherapy, the utilization of radiotherapy in the management of hepatocellular carcinoma was limited by toxicity, given that the whole liver was necessarily encompassed in the radiation portal. Radiation-induced veno-occlusive liver disease (classic RILD) was the dose-limiting complication, which was pathologically first described by Reed and Cox in the early 1960s (Reed 1966).
The advent of conformal radiotherapy permitted delineation of target lesions, thereby enabling partial volumes of the liver to be spared from radiotherapy. Furthermore, the dose-volume relationship between liver irradiation and RILD became better elucidated. In 2002, a Normal Tissue Complication Probability (NTCP) model was described which estimated a 50 % complication risk of RILD for whole-organ irradiation of 39.8 Gy for patients with primary hepatobiliary cancer. (Dawson and Normolle et al. 2002).
One of largest experiences of conformal radiotherapy in the treatment of hepatocellular carcinoma is derived from the Seong experience in Korea, which is a retrospective series of 298 patients with hepatocellular carcinoma. The majority (81.9 %) of patients were treated with conformal radiotherapy to a total dose of ≥45 Gy. On multivariate analysis, a BED of >53.1 Gy was shown to be a significant factor for better prognosis (Seong and Lee et al. 2009).
In the United States, the University of Michigan conducted a series of trials with conventionally fractionated, conformal partial liver radiotherapy which was delivered in doses up to 90 Gy in 1.5-Gy fractions on a twice-daily schedule with concurrent intra-arterial hepatic fluorodeoxyuridine (Robertson and Walker et al. 1997; McGinn and Ensminger et al. 1998; Dawson and McGinn et al. 2000; Ben-Josef and Normolle et al. 2005). For the 35 patients enrolled in these trials with hepatocellular carcinoma, an objective response was obtained in 56 % of patients, and median survival was 15.2 months. There was a statistically significant effect for dose, as patients who received a total dose of ≥75 Gy enjoyed improved progression free survival than patients who received lesser total radiotherapy doses (Ben-Josef and Normolle et al. 2005). A summary of trials of conformal radiotherapy is provided in Table 3. Typically, local control has been reported between 54 and 81 %, and 1-year overall survival ranges between 43 and 65 %.
Table 3
Trials of conformal, conventionally fractionated radiotherapy for Hepatocellular Carcinoma
Study | Patients | CP Class A (%) | Total dose (dose per fraction) | 1-year local control | 1-year overall survival |
|---|---|---|---|---|---|
Liu and Li et al. 2004 (Liu and Li et al. 2004) | 44 | 86 | 40–60 Gy, fractional dose unreported | 61 % | 61 |
Ben-Josef and Normolle et al. 2005 (Ben-Josef and Normolle et al. 2005) | 35 | 100 | 40–90 Gy in 1.5 Gy fx BID | 81 % | 57 |
Liang and Zhu et al. 2005 (Liang and Zhu et al. 2005) | 128 | 84 | 36–68 Gy, majority 4- to 6-Gy fx | 69 % at 3 months | 65 |
Kim and Kim et al. 2006 (Kim and Kim et al. 2006) | 70 | 88 | 44–54 Gy in 2- to 3-Gy fx | 54 % | 43 |
Mornex and Girard et al. 2006 (Mornex and Girard et al. 2006) | 27 | 59 | 36–66 Gy in 2-Gy fx | 78 % | NA |
Seong and Lee et al. 2009 (Seong and Lee et al. 2009) | 398 | 77 | 25–60 Gy, majority 1.8- to 5-Gy fx | NA | 45 |
2.2.6 Stereotactic Body Radiotherapy in the Management of Hepatocellular Carcinoma
Stereotactic body radiation therapy (SBRT) has emerged as powerful, non-invasive ablative technique with the capacity for local tumor eradication in the absence of surgery. The experience of SBRT for hepatocellular carcinoma is relatively small, given the new application of SBRT in the treatment of this entity. Several trials have reported their experiences with hepatocellular carcinoma and SBRT, with total doses typically between 36 and 60 Gy delivered in 3–6 fractions (Table 4) (Liang and Zhu et al. 2006; Mendez-Romero and Wunderink et al. 2006; Tse and Hawkins et al. 2008; Cardenes and Price et al. 2010; Goyal and Einstein 2010; Kwon and Bae et al. 2010; Louis and Dewas et al. 2010; Seo and Kim et al. 2010). In contrast to patients with metastatic liver tumors, the majority of patients with hepatocellular carcinoma have cirrhotic livers, so estimation of post-treatment hepatic function is increasingly important. Further details regarding the toxicity of SBRT in hepatocellular carcinoma are reviewed later in the “Toxicity” section.
Table 4
Studies of stereotactic body radiotherapy for Hepatocellular Carcinoma
Study | Patients | CP Class A (%) | Total dose (fractions) | 1-year local control (%) | 1-year overall survival (%) |
|---|---|---|---|---|---|
Mendez-Romero and Wunderink et al. 2006 (Mendez-Romero and Wunderink et al. 2006) | 8 | 75 | 37.5 Gy (3 fractions) for < 4 cm 25 Gy (5 fractions) or 30 Gy (3 fractions) for ≥ 4 cm | 75 | 75 |
Tse and Hawkins et al. 2008 (Tse and Hawkins et al. 2008) | 31 | 100 | 24–54 Gy (6 fractions) | 65 | 48 |
Cardenes and Price et al. 2010 (Cardenes and Price et al. 2010) | 17 (25 tumors) | 35 | 36–48 Gy (3 fractions) for CPA 36–42 Gy (3 fractions) or 40 Gy (5 fractions) for CPB | 100 | 75 |
Goyal and Einstein et al. 2010 (Goyal and Einstein et al. 2010) | 6 | NA | 24–45 Gy (1–3 fractions) | 100 | 67 |
Seo and Kim et al. 2010 (Seo and Kim et al. 2010) | 38 | 89 | 33–57 Gy (3–4 fractions) | 79 | 68 |
Kwon and Bae et al. 2010 (Kwon and Bae et al. 2010) | 42 | 90 | 30–39 Gy (3 fractions) | 72 | 93 |
Louis and Dewas et al. 2010 (Louis and Dewas et al. 2010) | 25 | 88 | 45 Gy (3 fractions) | 95 | 79 |
Stenmark and Liu et al. 2011 (Stenmark and Liu et al. 2011, January 20–22) | 31 | 69 | Majority 50 Gy (5 fractions) or 60 Gy (3 fractions) | 88 | 81 |
One of the largest prospective trials of SBRT in hepatocellular carcinoma was conducted at the Princess Margaret Hospital. In this phase 1 trial, 31 patients with unresectable hepatocellular carcinoma with Child-Pugh A liver function were treated with 6 fraction SBRT. The total SBRT dose was dependent upon NTCP calculations that estimated the risk of RILD from 5 to 20 % based on the University of Michigan experience. The median tumor dose was 36 Gy (range 24–54 Gy). A median overall survival of 11.7 months was observed for patients with hepatocellular carcinoma. There was a 24 % rate of Grade 3 transaminitis, and no acute Grade 4–5 toxicities. There was one late Grade 5 toxicity resulting from a GI bleed (Tse and Hawkins et al. 2008).
2.2.7 Chemotherapy in the Management of Hepatocellular Carcinoma
Sorafenib is an oral multikinase inhibitor of vascular endothelial growth factor and platelet-derived growth factor receptors. The SHARP (Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol) trial was a large phase 3, randomized, placebo-controlled trial that evaluated sorafenib in patients with advanced hepatocellular carcinoma (Llovet and Ricci et al. 2007). Patients receiving sorafenib experienced a 3 month improvement in median survival than those randomized to placebo (10.7 months vs 7.9 months, P < 0.001). Time to radiographic progression was also significantly improved with the addition of sorafenib by approximately 3 months. Similar in design to the SHARP trial, the Asia–Pacific trial found a statistically significant improvement in overall survival (6.5 months vs 4.2 months) as well as median time to progression (2.8 months vs 1.4 months) for patients treated with sorafenib versus placebo (Cheng and Kang et al. 2009). The NCCN designates sorafenib as the only treatment option with a category 1 designation for Child-Pugh Class A patients with unresectable disease (Benson and Abrams et al. 2009).
3 Metastatic Liver Disease
3.1 Clinical Factors
The spectrum of metastatic disease to the liver is broad, ranging from a single metastatic lesion to innumerable hepatic metastases. Traditionally, the presence of liver metastases denoted an incurable state, and interventions were largely restricted to systemic therapy and/or palliative in nature. However, surgical series of metastasectomy have challenged this assumption, with recent studies documenting 5-year survival rates from 42 to 71 % for patients with solitary hepatic metastases (Aloia and Vauthey et al. 2006; Simmonds and Primrose et al. 2006; Taniai and Yoshida et al. 2006; Tomizawa and Ohwada et al. 2006; Wei and Grant et al. 2006). From these observations, the concept of ‘oligometastases’ denoted that, for patients with limited volume and sites of metastatic disease, localized cancer therapies have the potential to be curative in nature (Weichselbaum and Hellman 2011).
The identification of patients with hepatic metastases who have disease that is potentially amenable to curative, locoregional therapies has been investigated in a retrospective manner by surgical series. There is evidence that patients presenting synchronously with a primary colon cancer and liver metastases may have a more disseminated disease state and corresponding shorter disease-free interval than for patients developing metachronous metastases (Tsai and Su et al. 2007). Fong et al. examined the clinical and pathologic findings of 1,001 consecutive patients at a single institution undergoing partial liver resection for metastatic colorectal disease. Five-year survival for all patients was 37 %, and the 10-year survival was 22 %. Findings that portended a poor long-term survival on multivariate analysis included positive surgical margins, extrahepatic disease, node positive primary, disease-free interval of <12 months, >1 hepatic metastasis, largest hepatic tumor >5 cm, and CEA > 200 mg/mL (Fong and Fortner et al. 1999). A clinical risk score corresponding to tumor recurrence at 1, 2, 3, 4, and 5 years was developed from these findings (Table 5).
Table 5
Clinical risk score for tumor recurrence
Survival (%) | ||||||
|---|---|---|---|---|---|---|
Score | 1 year | 2 year | 3 year | 4 year | 5 year | Median (mo) |
0 | 93 | 79 | 72 | 60 | 60 | 74 |
1 | 91 | 76 | 66 | 54 | 44 | 51 |
2 | 89 | 73 | 60 | 51 | 40 | 47 |
3 | 86 | 67 | 42 | 25 | 20 | 33 |
4 | 70 | 45 | 38 | 29 | 25 | 20 |
5 | 71 | 45 | 27 | 14 | 14 | 22 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree