, Foster D. Lasley2, Indra J. Das2, Marc S. Mendonca2 and Joseph R. Dynlacht2
(1)
Department of Radiation Oncology, CHRISTUS St. Patrick Regional Cancer Center, Lake Charles, LA, USA
(2)
Department of Radiation Oncology, Indiana University School of Medicine, Indianapolis, IN, USA
Genetic Changes in Cancer
Analysis of cancer cells shows that most of them have highly abnormal DNA, with multiple changes compared to healthy cells.
As discussed in Chapt. 17, many different types of mutation may occur.
Mutations occur through several mechanisms:
Heritable: Present at birth.
BRCA1/2 mutations cause heritable breast/ovarian cancer.
FAP and MSH/MLH mutations cause heritable colon cancer.
Rb mutations cause heritable Retinoblastoma and soft tissue sarcomas.
p53 mutations cause Li-Fraumeni syndrome, with multiple malignancies.
Spontaneous: Random mutations due to aging, oxidation, and the process of DNA replication and mitosis.
Genomic instability: Loss of DNA repair and apoptosis pathways leads to accumulation of spontaneous mutations over time.
Chemical induced: Many chemicals (tobacco smoke, etc.) cause base damage or DNA crosslinks, and are more likely to cause point mutations.
Radiation induced: Radiation causes double strand breaks and is more likely to cause gross mutations.
Cell fusion: Two cells combine into one, doubling DNA content (tetraploidy) and increasing the rate of mutation.
Abnormal genes may also be introduced by a virus:
HPV is the widely-publicized virus responsible for cervical cancers and some head and neck cancers.
EBV is responsible for nasopharyngeal cancers and Burkitt lymphoma.
Epigenetic Changes in Cancer
As described in Chapt. 17, epigenetic changes are changes in gene function without any change in the gene itself.
The most common epigenetic change is chromatin modification.
Methylation decreases gene expression.
Acetylation increases gene expression.
Hyper-methylation is the most common form of epigenetic silencing.
Cancer cells use methylation to turn off tumor suppressor and DNA repair genes. This may leave them vulnerable to DNA damaging agents (chemo, radiation).
MGMT methylation in glioblastoma correlates with response to temozolomide.
Multi-Step Model of Carcinogenesis
Cancer is a disease of uncontrolled proliferation, invasion, and metastasis. It usually takes many changes to turn a normal cell into a cancer cell.
A cell with uncontrolled proliferation but no invasion or metastasis may form a tumor, but it is likely to be benign or pre-malignant.
For example, genital warts are typically benign, but with high-risk HPV subtypes they may become malignant.
Initiation is the first mutation(s) that promotes uncontrolled proliferation.
Promotion is a second mutation(s) that has little effect in a normal cell, but after initiation can cause a further increase in proliferation.
Progression can lead to additional mutations that confer malignant characteristics such as invasion and metastasis (Fig. 18.1).
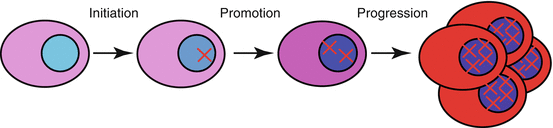
Fig. 18.1
Multiple mutations are required to turn a normal cell into a cancer cell. These are referred to as initiation, promotion, and progression.
Genetic analysis of benign, premalignant and malignant tumors shows that mutations frequently happen in a specific order:
For example, APC and K-Ras are commonly mutated in benign colon adenomas. (initiation).
CIN and DCC are commonly mutated in dysplastic colon adenomas. (promotion).
p53 is commonly mutated in invasive colon cancer. (progression).
Clinical Significance of Cancer Genomics
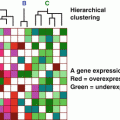
Gene profiling technologies have allowed for measurement of gene expression in human tumors; this is increasingly used for prognostic and therapeutic decision making.
Cytotoxic therapy (classical chemotherapy) damages DNA or inhibits metabolic pathways common to many human cells.
Genomics may be used to predict efficacy and toxicity of cytotoxic drugs.
Targeted therapy is designed to specifically inhibit certain cell signaling pathways.
Genomics may be used to identify specific mutations or molecular pathways that can be targeted by drugs.
Not all targeted therapies target oncogenes.
Prognostic gene panels attempt to predict tumor behavior and treatment response.
The Oncotype DX multi-gene panel is used to predict the utility of chemotherapy in early stage invasive breast cancer.
The Oncotype DCIS multi-gene panel may predict the utility of whole-breast irradiation in DCIS.
Oncogenes and Tumor Suppressors