Gastric Carcinoma
There has been a dramatic decline in the incidence of gastric carcinoma since the late 1940s. Nevertheless, it remains a deadly disease, with overall 5-year survival rates of less than 20%. Since the 1980s, attention has been focused on the role of double-contrast barium studies and endoscopy for the early diagnosis of gastric cancer. The Japanese have had tremendous success in detecting early gastric cancer by mass screening of the adult population with these techniques. However, it is difficult to justify such screening programs outside Japan because of the lower incidence of this malignancy. Thus, the prognosis for gastric carcinoma remains dismal in most parts of the world.
Epidemiology
Gastric carcinoma has striking geographic variations, with the highest incidences reported in Japan, Chile, Finland, Poland, and Iceland. However, Japanese immigrants and their offspring living in the United States have a significantly lower incidence of gastric cancer than those living in Japan. Such epidemiologic data suggest that environmental factors have a major role in the development of gastric carcinoma. Dietary habits may be particularly important in explaining the observed geographic differences in cancer risk. Helicobacter pylori infection of the stomach has also increasingly been implicated as a major risk factor in the development of gastric carcinoma in various parts of the world. Other predisposing conditions include atrophic gastritis, pernicious anemia, gastric polyps, partial gastrectomy, Ménétrier’s disease, and hereditary factors. These risk factors for gastric cancer are discussed separately in the following sections.
Dietary Factors
Studies have shown that diets rich in salted, smoked, or poorly preserved foods are associated with an increased risk of gastric cancer, whereas diets rich in fruits and vegetables are associated with a decreased risk. Foods containing nitrates or nitrites have also been implicated in the development of gastric cancer. These compounds are converted by bacteria to nitrosamines, which are thought to have a carcinogenic effect on the stomach. Thus, populations with a higher average intake of nitrates and nitrites probably have a higher gastric cancer risk. Conversely, vitamin C (ascorbic acid) appears to have a protective effect by reducing nitrites to nitric oxide and preventing the formation of nitrosamine compounds. This could explain why the consumption of fruit and vegetables is associated with a lower risk of developing gastric cancer. One study also found that a high intake of cereal fiber is associated with a significantly lower risk of carcinoma of the cardia, possibly because of the nitrite-scavenging properties of cereal fiber.
Helicobacter pylori Gastritis
H. pylori has increasingly been implicated as a major risk factor in the pathogenesis of gastric carcinoma; H. pylori is thought to be the leading cause of gastric cancer worldwide. Various studies have found that people with H. pylori gastritis have a two to six times greater risk of developing gastric cancer than those without this infection. It has been well documented that people with long-standing H. pylori gastritis are more likely to develop atrophic gastritis. Over a period of years, chronic atrophic gastritis may progress to gastric atrophy, intestinal metaplasia, dysplasia, and, eventually, gastric carcinoma. Other studies have found that people with H. pylori gastritis are more likely to develop the intestinal type of gastric carcinoma than people without this infection (see later, “ Gastric Carcinoma: Pathology ”). It has also been shown that certain strains of H. pylori , which produce a vacuolating cytotoxin, cytotoxin-associated gene A (cagA) , are associated with a higher prevalence of atrophic gastritis and gastric carcinoma. A meta-analysis of 16 studies from the literature found that infection with cagA -positive strains of H. pylori substantially increased the risk of gastric cancer compared to the risk associated with H. pylori infection irrespective of cagA status.
Nevertheless, gastric cancer develops in only a tiny percentage of all people with H. pylori gastritis, so other genetic or environmental factors presumably have a role in cancer pathogenesis. In a cost-effectiveness model, Parsonnet and colleagues found that widespread screening and treatment of adults for H. pylori is a potentially cost-effective strategy for the prevention of gastric cancer, particularly in high-risk populations. However, further investigation is needed to determine whether such an approach is justified.
Atrophic Gastritis
Atrophic gastritis has been classified into two types, which have different histologic, immunologic, and secretory characteristics. Type A gastritis is usually associated with pernicious anemia (see next section). In contrast, type B gastritis (which is more common) predominantly involves the antrum and usually results from mucosal injury by H. pylori or, less commonly, by other toxic agents (see Chapter 30 ). Long-term studies indicate that about 10% of patients with type B gastritis develop gastric carcinoma within 10 to 20 years. It has been postulated that chronic atrophic gastritis leads to the development of intestinal metaplasia, dysplasia, and, eventually, adenocarcinoma. This pathologic sequence of events is supported by the frequent association of gastric carcinoma and intestinal metaplasia on surgical specimens. Thus, chronic atrophic gastritis and intestinal metaplasia are thought to be important precursor conditions for the development of gastric carcinoma.
Pernicious Anemia
Type A gastritis predominantly involves the gastric fundus and body and is usually thought to result from immunologic injury by antiparietal cell antibodies in patients with pernicious anemia. This type of gastritis may also be associated with an increased risk of gastric cancer. In various studies, the incidence of gastric cancer in patients with pernicious anemia has been two to three times greater than that expected for the general population. Most such tumors involve the gastric fundus or body. Although some investigators advocate radiologic or endoscopic surveillance of patients with known pernicious anemia, others believe that the cancer risk is not high enough to warrant routine surveillance. Nevertheless, any patient with pernicious anemia who has guaiac-positive stool or other upper gastrointestinal (GI) complaints should be aggressively evaluated for possible gastric carcinoma.
Gastric Polyps
Adenomatous polyps account for less than 20% of all gastric polyps. Despite their rarity, these polyps are premalignant lesions that are capable of undergoing malignant degeneration via an adenoma-carcinoma sequence similar to that found in the colon. Nearly 50% of gastric adenomas larger than 2 cm are found to harbor carcinomatous foci. All adenomatous polyps should therefore be resected because of the risk of malignant transformation. Nevertheless, adenocarcinoma is about 30 times more common than adenomatous polyps in the stomach, so most gastric cancers are thought to originate de novo and not from preexisting polyps.
Partial Gastrectomy
Patients who undergo partial gastrectomy appear to be at increased risk for the development of gastric carcinoma. A gastric stump cancer is defined as a primary carcinoma of the gastric remnant occurring a minimum of 5 years after partial gastrectomy for gastric ulcers or other benign disease. Affected individuals have usually undergone a Billroth II rather than a Billroth I procedure. These tumors tend to be located in the distal portion of the gastric remnant near the gastrojejunal anastomosis. It has been postulated that recurrent bile reflux above the anastomosis causes chronic gastritis, intestinal metaplasia, and, eventually, gastric carcinoma. In various studies, the mortality from gastric cancer 15 years or more after partial gastrectomy has been three to seven times greater than that expected for the general population. Some authors therefore advocate routine endoscopic surveillance of the gastric remnant starting 15 years after surgery. However, other investigators have found no greater incidence of gastric carcinoma than that expected for the general population as long as 25 years after surgery. Thus, the need for surveillance in these patients remains controversial.
Gastric carcinoma has also been reported as a late complication of gastrojejunostomy for benign disease in the absence of partial gastrectomy. As in patients who have undergone a Billroth II procedure, bile reflux gastritis and intestinal metaplasia are thought to be predisposing factors. Whatever the explanation, the risk of developing gastric carcinoma also appears to be increased in patients who undergo a gastrojejunostomy without a gastric resection.
Ménétrier’s Disease
Ménétrier’s disease is a rare disorder of unknown etiology characterized by a hypertrophic gastropathy associated with decreased gastric acid secretion and a protein-losing enteropathy (see Chapter 30 ). Anecdotal cases of gastric carcinoma have been described in patients with Ménétrier’s disease. However, it is unclear whether this association is coincidental or whether Ménétrier’s disease is a premalignant condition.
Hereditary Factors
Hereditary factors have also been implicated in the development of gastric cancer because a positive family history has been associated with an increased risk of this malignancy. In one study, the prevalence of H. pylori gastritis was significantly higher in subjects who had parents with gastric cancer than in other subjects. Such findings suggest that familial aggregation of gastric cancer may be explained at least partly by familial clustering of H. pylori gastritis. Patients with gastric cancer also have been found to have a higher frequency of blood type A and a lower frequency of blood type O than the general population.
Pathology
Gross Features
Most gastric carcinomas are polypoid or ulcerated lesions. Polypoid carcinomas have a plaquelike, lobulated, or fungating appearance. Ulcerated carcinomas may contain deep, irregular or broad, shallow areas of ulceration caused by necrosis and excavation of the tumor. The ulcer may be surrounded by a thin rind of malignant tissue or by an obvious mass lesion, so many polypoid tumors have ulcerated components. Less commonly, gastric carcinomas may be diffusely infiltrative lesions that spread along the gastric wall with relatively little intraluminal growth. These scirrhous tumors may produce a classic linitis plastica appearance because of submucosal thickening and fibrosis incited by the tumor. Other gastric carcinomas may be superficial spreading lesions that are confined to the mucosa or submucosa without invading the deep muscle layers of the gastric wall.
Rarely, patients with gastric carcinoma have multiple primary lesions separated by normal intervening mucosa. In various series, two or more synchronous tumors have been found in 2% to 8% of all patients with gastric cancer. In such cases, individual lesions may have different morphologic features.
Histologic Features
More than 95% of malignant neoplasms in the stomach are adenocarcinomas. The remaining lesions consist of lymphoma, gastrointestinal stromal tumors (GISTs), Kaposi’s sarcoma, carcinoid, and other rare malignant tumors (see Chapter 33 ). Gastric carcinomas may be classified into two types: (1) an intestinal type characterized by well-formed glandular structures that tend to grow in a fungating manner; and (2) a diffuse type characterized by poorly cohesive cells that tend to infiltrate and thicken the gastric wall without producing a discrete mass. Intestinal-type lesions are more likely to involve the distal stomach and are often associated with underlying atrophic gastritis. In contrast, diffuse-type lesions are more likely to involve the entire stomach (especially the gastric cardia) and tend to have a worse prognosis.
Gastric carcinomas have been further classified by the World Health Organization into the following subtypes—papillary, tubular, mucinous, and signet ring cell. Most tumors that are capable of forming glandular structures secrete mucinous substances and, occasionally, excessive mucin accumulates extracellularly in colloid or mucinous adenocarcinomas. Other adenocarcinomas are composed of distinctive signet ring cells containing large amounts of intracytoplasmic mucin and compressed, eccentric nuclei. As they infiltrate the gastric wall, these signet ring cells often incite a desmoplastic response in the submucosa and muscularis propria, producing the classic pathologic features of a primary scirrhous carcinoma. In various series, scirrhous tumors have been found to account for 5% to 15% of all gastric cancers.
Most adenocarcinomas of the stomach are diagnosed at an advanced stage. By definition, advanced gastric cancers have already invaded the muscularis propria. These tumors are usually associated with metastases to regional lymph nodes or other local or distant structures. In contrast, early gastric cancers are defined histologically as cancers in which malignant invasion is limited to the mucosa or submucosa, regardless of the presence or absence of lymph node metastases. The largest number of early gastric cancers has been reported in Japan as a result of mass screening of the adult population. Unlike advanced carcinomas, which have a dismal prognosis, early gastric cancers are curable lesions with 5-year survival rates greater than 90% (see later, “ Treatment and Prognosis ”).
Distribution
At one time, most gastric carcinomas were located in the antrum. Since the late 1940s, however, there has been a gradual shift in the distribution of gastric cancer from the antrum proximally to the body and fundus. This changing distribution has been attributed primarily to a rising incidence of carcinoma of the gastric cardia, which has increased at a rate exceeding that of any other cancer. As a result, these tumors now have a relatively even distribution in the stomach, with about 30% located in the antrum, 30% in the body, and 40% in the fundus or cardiac region. This changing pattern of disease has important implications for cancer detection because the gastric cardia and fundus must be carefully evaluated for signs of malignancy in all patients who undergo barium studies or endoscopy to rule out gastric carcinoma.
Routes of Spread
Gastric carcinoma may invade local, regional, or distant structures by four pathways—direct extension, lymphatic spread, intraperitoneal seeding, and hematogenous metastases. The various pathways of spread are discussed separately in the following sections.
Direct Extension
Gastric carcinoma has a tendency to invade adjacent structures such as the liver, pancreas, and spleen. Longitudinal spread of tumor along the GI tract is also relatively common. The distal esophagus is directly involved by carcinoma of the cardia in about 60% of patients, whereas the duodenum is involved by carcinoma of the antrum in 5% to 25% of patients. Tumor involving the greater curvature of the stomach may also spread inferiorly via the gastrocolic ligament to the transverse colon, occasionally resulting in the development of a gastrocolic fistula.
Lymphatic Spread
Because of the abundant lymphatics in the stomach, lymph node metastases are found in 74% to 88% of patients with gastric carcinoma. These patients may initially have involvement of local (perigastric) nodes and, subsequently, regional (celiac, hepatic, left gastric, and splenic) or distant (left supraclavicular and left axillary) nodes. The frequency of lymphatic metastases is related to the size and depth of penetration of the tumor. Nevertheless, lesions are still classified histologically as early gastric cancers, regardless of the presence of local lymph node metastases, if malignant invasion is confined to the mucosa or submucosa.
Intraperitoneal Seeding
Patients with advanced gastric carcinoma may develop malignant ascites, resulting in intraperitoneal-seeded or omental metastases. Diffuse carcinomatosis may also lead to the development of small bowel obstruction. Some patients with signet ring cell adenocarcinomas have bilateral “drop” metastases to the ovaries, known as Krukenberg tumors. Although other malignant tumors can also have drop metastases to the ovaries, gastric carcinoma is responsible for most cases. Some patients with gastric carcinoma may present with bilateral ovarian masses as the initial manifestation of their disease.
Hematogenous Metastases
Because the stomach is drained by the portal vein, the liver is the most common site of hematogenous (blood-borne) metastases from gastric carcinoma. Other less common sites of hematogenous spread include the lungs, adrenals, kidneys, bones, and brain.
Clinical Findings
Gastric carcinoma is usually considered a disease of middle and late life, with a peak incidence between 50 and 70 years of age. However, 3% to 5% of patients with gastric cancer are less than 35 years of age and 1% are less than 30 years of age. The percentage of young patients with gastric cancer has more than doubled since 1970. For reasons that are unclear, these young people tend to have more aggressive lesions that are associated with a worse prognosis than most gastric cancers.
Gastric carcinoma is twice as common in men as in women. The male predilection of these tumors is even greater for carcinoma of the cardia, which is seven times more likely to occur in men than in women. This explanation for this discrepancy is unclear.
Most patients with gastric carcinoma are symptomatic only when they have advanced tumors with local or distant metastases. The most common presenting findings include epigastric pain, bloating, early satiety, nausea, vomiting, dysphagia, anorexia, weight loss, and signs or symptoms of upper GI bleeding, such as hematemesis, melena, guaiac-positive stool, and iron deficiency anemia. However, similar findings may be caused by ulcers, gastritis, or other benign conditions. As a result, there is often a considerable lag between the onset of symptoms and the diagnosis of gastric cancer.
The clinical presentation is also affected by the location and morphologic features of the tumor. For example, nausea and vomiting are common findings in patients with obstructing lesions involving the distal antrum or pyloric region. In contrast, patients with scirrhous carcinomas may develop early satiety because of the decreased compliance of a stomach that is diffusely infiltrated by tumor. Other patients with carcinoma of the cardia may present with recent onset of dysphagia caused by tumor obstructing the cardia. Some individuals with dysphagia may complain of food sticking behind the sternum, whereas others may have a sensation of blockage referred upward to the thoracic inlet or even the pharynx. The gastric cardia and fundus should therefore be carefully evaluated in all patients with dysphagia, regardless of the subjective site of obstruction, to rule out a carcinoma of the cardia masquerading as a esophageal or pharyngeal disorder.
Some patients with advanced gastric cancer may initially present with signs or symptoms of metastatic disease, such as anorexia, weight loss, abdominal masses, hepatic enlargement, jaundice, ascites, back pain, or neurologic findings. Patients with ovarian metastases may have bilateral pelvic masses (so-called Krukenberg tumors), and patients with drop metastases to the rectosigmoid colon may have a palpable Blumer’s shelf on rectal examination.
Endoscopic Findings
When brushings and biopsy specimens are obtained, endoscopy has a reported overall sensitivity of 94% to 98% in the diagnosis of gastric carcinoma. However, multiple biopsy specimens should be taken from suspicious lesions to decrease the risk of sampling error. It should also be recognized that endoscopy is a much less reliable technique for diagnosing scirrhous tumors than other gastric carcinomas. In various series, the sensitivity of endoscopic brushings and biopsy specimens in detecting these lesions has ranged from only 33% to 70%. False-negative histologic findings may occur not only because these scirrhous carcinomas are located predominantly in the submucosa, but also because the tumor cells are often separated by sheets of fibrosis. In some cases, multiple endoscopic examinations may be required for a definitive histologic diagnosis. Thus, excessive reliance on negative endoscopic biopsy specimens may cause inordinate delays in the treatment of these patients. Finally, the presence and extent of tumor is often underestimated on the basis of the gross endoscopic findings because the mucosa overlying these lesions usually appears normal.
Radiographic Findings
Early Gastric Cancer
The double-contrast upper GI examination has been widely recognized as the best radiologic technique for the diagnosis of early gastric cancer. The Japanese Endoscopic Society has divided these lesions into three basic types. Type I lesions are elevated lesions that protrude more than 5 mm into the lumen. Type II lesions are superficial lesions that are further subdivided into three groups—types IIa, IIb, and IIc—depending on their morphologic features. Type IIa lesions are elevated but protrude less than 5 mm into the lumen. Type IIb lesions are essentially flat. Type IIc lesions are slightly depressed but do not penetrate beyond the muscularis mucosae. Type III lesions are true mucosal ulcerations, with the ulcer penetrating the muscularis mucosae but not the muscularis propria. When early gastric cancers exhibit more than one of these morphologic features, they may have a dual classification, with the most predominant pattern listed first (e.g., type III + IIc).
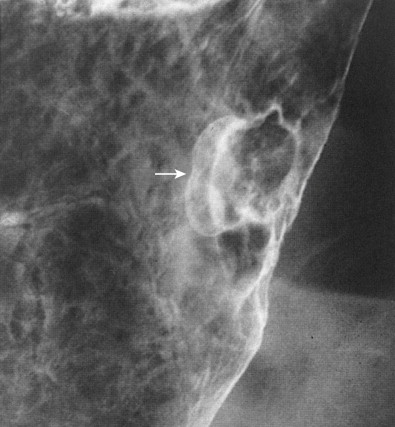
Type I early gastric cancers typically appear as small, elevated lesions in the stomach. Because adenomatous polyps may undergo malignant degeneration (see earlier, “ Gastric Polyps ”), the possibility of early gastric cancer should be suspected for any sessile or pedunculated polyps larger than 1 cm. Other type I lesions may protrude considerably into the lumen and still be classified histologically as early gastric cancers ( Fig. 32-1A ). Thus, polypoid carcinomas cannot be diagnosed definitively as early or advanced lesions on the basis of the radiographic findings.
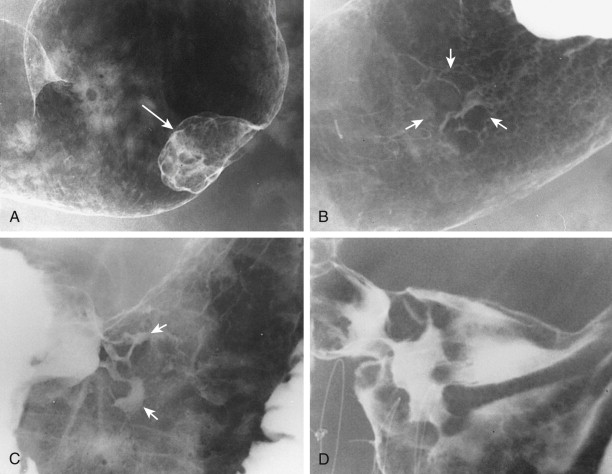
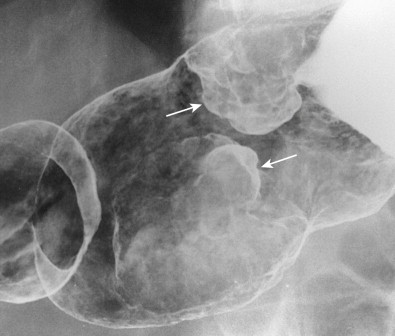
Type II early gastric cancers are superficial lesions with elevated (IIa), flat (IIb), or depressed (IIc) components. These lesions may be manifested by plaquelike elevations, mucosal nodularity, shallow areas of ulceration, or some combination of these findings ( Fig. 32-1B and C ). Occasionally, type II lesions may be quite extensive and involve a considerable surface area of the stomach.
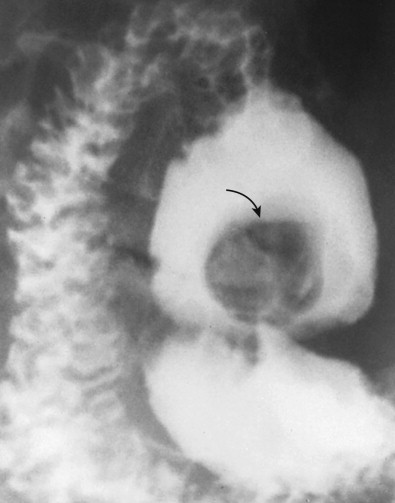
Type III early gastric cancers are characterized by shallow, irregular ulcer craters with nodularity of the adjacent mucosa and clubbing, fusion, or amputation of radiating folds ( Fig. 32-1D ). Careful analysis of the radiographic findings usually allows these lesions to be distinguished from benign gastric ulcers, which have different radiographic features (see Chapter 29 ). Although some lesions with an equivocal or suspicious appearance are found to be benign ulcers, endoscopy and biopsy should be performed for all lesions with suspicious radiographic findings to avoid missing early cancers.
About 70% of the ulcers in type IIc or III early gastric cancers are reported to undergo substantial healing on medical treatment. It has been postulated that these cancers are characterized by a cycle of ulceration, healing, and recurrent ulceration. Rarely, complete healing of malignant ulcers has also been described. However, malignant tumors should still be suspected on follow-up barium studies if mucosal nodularity or other abnormalities are detected at the site of the previous ulcer.
Japanese researchers have reported an incidence of early gastric cancer (i.e., the percentage of all gastric cancers detected as early lesions) of 25% to 46% compared with an incidence of only 5% to 24% in Western countries. This discrepancy can be attributed to mass screening of the adult population in Japan because of the unusually high prevalence of gastric carcinoma in that country. Occasionally, early gastric cancers may be detected in symptomatic patients with epigastric pain, upper GI bleeding, or other complaints. Early gastric cancers may also be discovered fortuitously in patients who undergo radiologic or endoscopic examinations for other reasons. Nevertheless, radiologists and endoscopists in the West are unlikely to detect a substantial number of early gastric cancers as long as these examinations are performed predominantly on symptomatic patients.
Advanced Carcinoma
Abdominal Radiographs.
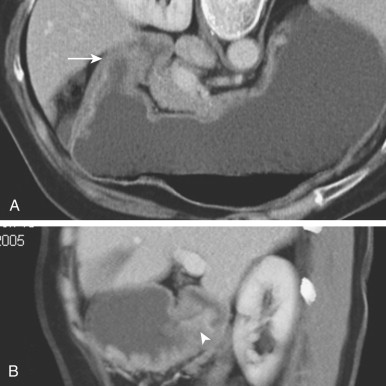
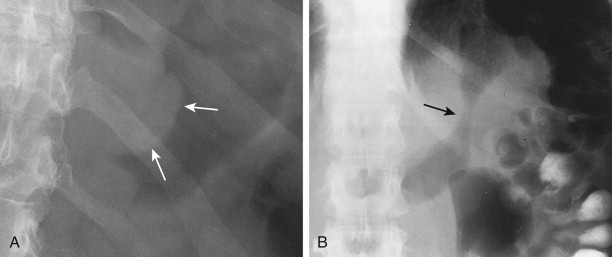
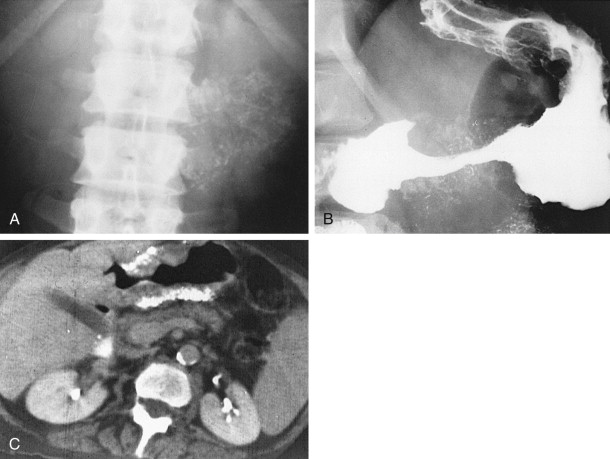
Polypoid gastric carcinomas are occasionally recognized on abdominal radiographs by the presence of a soft tissue mass indenting the gastric air shadow ( Fig. 32-2A ). Primary scirrhous carcinomas can also be recognized by a narrowed, tubular configuration of the gas-filled stomach ( Fig. 32-2B ). Rarely, mucin-producing scirrhous carcinomas may contain gross areas of calcification that have a stippled, punctate, or sandlike appearance ( Fig. 32-3A ). When abdominal radiographs are worrisome for gastric carcinoma, barium studies or computed tomography (CT) should be performed for a more definitive diagnosis ( Fig. 32-3B ). CT is a particularly sensitive technique for demonstrating the calcification in mucin-producing scirrhous tumors ( Fig. 32-3C ).
Barium Studies.
Accurate diagnosis of gastric cancer has always been an important goal of barium studies of the upper GI tract. Unfortunately, single-contrast examinations have an overall sensitivity of only 75% in diagnosing these tumors. In one study of 80 patients with gastric carcinoma, however, the lesion was detected on double-contrast examinations in 99% of cases, and malignant tumor was diagnosed or suspected on the basis of the radiographic findings in 96%. In the same study, it was found that endoscopy had been recommended because of radiographic findings that were equivocal or suggestive of tumor in only 4% of all patients who underwent double-contrast examinations during this period. Thus, a high sensitivity can be achieved in the diagnosis of gastric carcinoma on double-contrast studies without exposing an inordinate number of patients to unnecessary endoscopy. In another study, missed gastric cancers usually resulted from perceptual errors related to depressed lesions overlooked in the thin barium pool with compression or flow technique.
Advanced gastric carcinomas may appear as polypoid, ulcerative, or infiltrating lesions. However, many tumors have mixed morphologic features, so there is considerable overlap in the classification of these lesions. Because scirrhous carcinomas and cardiac carcinomas produce distinctive radiographic findings, these lesions are considered separately in later sections.
Polypoid carcinomas are lobulated or fungating masses that protrude into the lumen ( Figs. 32-4 and 32-5 ). On double-contrast studies, lesions on the dependent or posterior wall are seen as filling defects in the barium pool, whereas lesions on the nondependent or anterior wall are etched in white by a thin layer of barium trapped between the edge of the mass and adjacent mucosa. These tumors often contain one or more irregular areas of ulceration. Occasionally, polypoid antral carcinomas may prolapse through the pylorus into the duodenum, appearing as mass lesions at the base of the bulb ( Fig. 32-6 ). Rarely, two or more synchronous carcinomas may be present in the stomach (see Fig. 32-5 ).
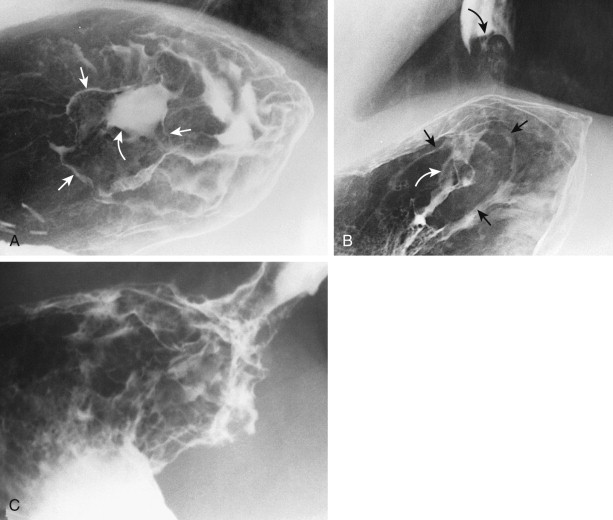
Ulcerated carcinomas are those in which the bulk of the tumor mass has been replaced by ulceration ( Figs. 32-7 and 32-8 ). Although these lesions are often called malignant ulcers, the term is a misnomer, because it is not the ulcer but the surrounding tumor that is malignant. In general, malignant ulcers are characterized en face by an irregular ulcer crater eccentrically located within a rind of malignant tissue. The ulcers may have scalloped, angular, or stellate borders. Discrete tumor nodules are often seen in the adjacent mucosa. Folds converging to the edge of the ulcer may be blunted, nodular, clubbed, or fused as a result of tumor infiltration. On double-contrast studies, malignant ulcers on the nondependent or anterior wall may be etched in white, so a double ring shadow is observed in the stomach, with the outer ring representing the edge of the tumor and the inner ring representing the edge of the ulcer (see Fig. 32-7A ). In such cases, prone compression views should demonstrate filling of the ulcer crater within a discrete tumor mass on the anterior wall (see Fig. 32-7B ). A biphasic examination is therefore essential for proper interpretation of these lesions.
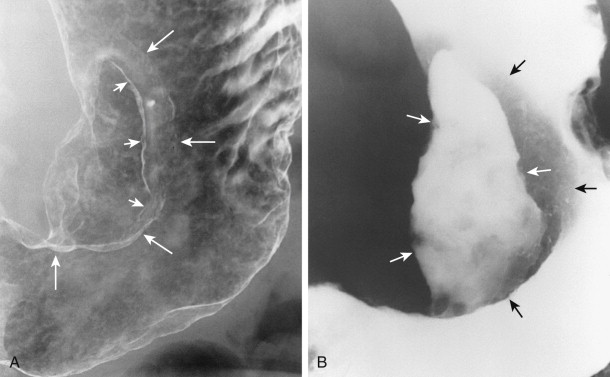
When viewed in profile, malignant ulcers usually have an intraluminal location, often within a discrete tumor mass (see Figs. 32-8B and C ), whereas benign ulcers project beyond the adjacent contour of the stomach. However, this criterion can be used only for ulcers on or near the lesser or greater curvature. The tumor mass surrounding malignant ulcers usually forms acute angles with the adjacent gastric wall rather than the obtuse, gently sloping angles expected for a benign mound of edema. Clubbed or nodular folds may also be seen radiating to the edge of the ulcer crater or to the edge of the surrounding mass as a result of tumor infiltrating these folds (see Fig. 32-8B ).
No sign in GI radiology has generated more confusion than the meniscus sign of a malignant ulcer, which was originally described by Carman in 1921 and refined by Kirklin in 1934. The Carman-Kirklin meniscus complex is caused by a cancer straddling the lesser curvature of the gastric antrum or body, in which the tumor is a broad, flat lesion with central ulceration and elevated margins. Careful compression of the lesion may reveal a meniscoid ulcer with a convex inner border and a concave outer border that does not project beyond the expected gastric contour (see Figs. 32-7B and 32-8A ). A radiolucent halo may be seen abutting the meniscus because of apposition of the elevated edges of the tumor on the anterior and posterior walls. Although the Carman-Kirklin meniscus complex is a reliable radiologic sign of malignancy, it can be demonstrated in only a small percentage of all patients with malignant ulcers.
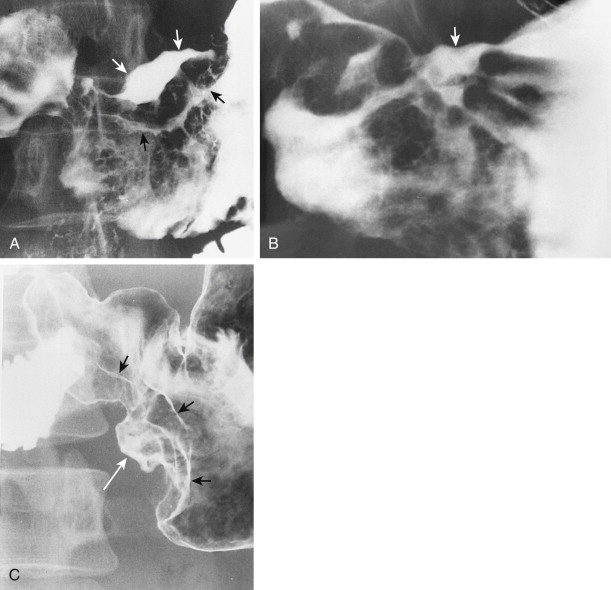
Infiltrating carcinomas are manifested by irregular narrowing of the stomach with nodularity and spiculation of the mucosa ( Fig. 32-9 ). Some infiltrating lesions may have polypoid or ulcerated components. In advanced cases, these lesions may cause gastric outlet obstruction.
Transpyloric spread of antral carcinoma into the duodenum can be demonstrated on barium studies in 5% to 25% of patients. Duodenal involvement is manifested by mass effect, nodularity, ulceration, or irregular narrowing of the proximal duodenum. Although transpyloric spread of tumor occurs in a higher percentage of patients with gastric lymphoma, gastric carcinoma is more likely to produce this finding because of its higher incidence.
Rarely, advanced gastric carcinomas on the greater curvature of the stomach may spread inferiorly via the gastrocolic ligament to the superior border of the transverse colon, resulting in the development of a gastrocolic fistula. Although these fistulas may occasionally be shown on an upper GI study, they are more likely to be demonstrated on a barium enema because of the higher pressures generated during this examination.
Scirrhous Carcinoma
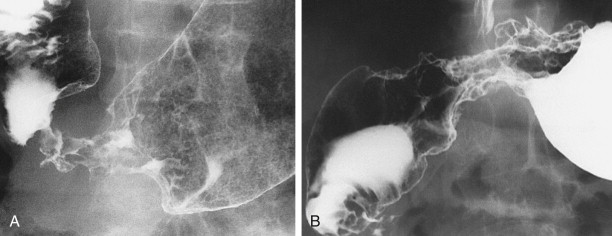
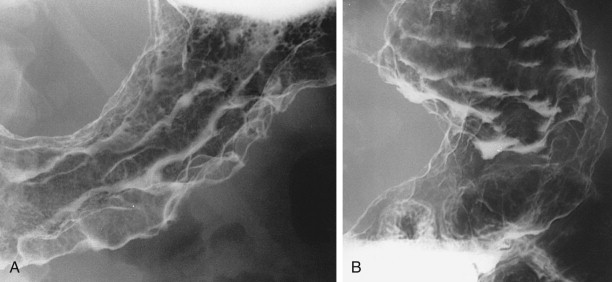
Scirrhous gastric carcinomas are traditionally thought to involve the distal half of the stomach, arising near the pylorus and gradually extending upward from the antrum into the body and fundus. These tumors are classically manifested on barium studies by irregular narrowing and rigidity of the stomach, producing a linitis plastica (“leather bottle”) appearance ( Fig. 32-10 ). In advanced cases, the stomach may be diffusely infiltrated by tumor (see Fig. 32-10B ). Other patients may have localized scirrhous tumors confined to the prepyloric region of the antrum, appearing as short, annular lesions with shelflike proximal margins ( Fig. 32-11 ). With double-contrast technique, however, 20% to 40% of patients with scirrhous tumors are found to have localized lesions involving the gastric fundus or body with sparing of the antrum ( Figs. 32-12 and 32-13 ). Detection of these lesions has presumably improved because of better gaseous distention of the proximal stomach on double-contrast studies. Whatever the explanation, radiologists should be aware that a large percentage of scirrhous carcinomas are localized lesions involving the gastric fundus or body rather than the classic form of linitis plastica involving the distal stomach.
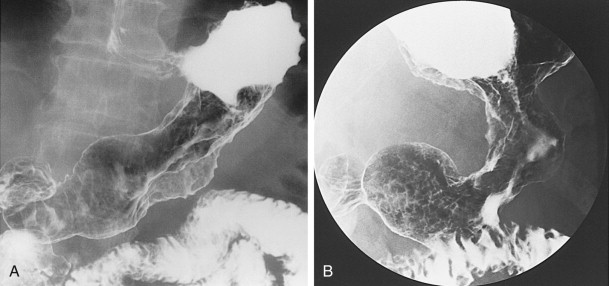
Because of intense fibrosis incited by scirrhous gastric carcinomas, these tumors may convert the stomach into a narrowed, rigid tube that loses its normal compliance and distensibility. As a result, barium paradoxically may empty from the stomach more rapidly than normal, so all the ingested barium enters the duodenum and proximal small bowel within minutes of starting the examination. This same phenomenon accounts for the early satiety that develops in some patients because the narrowed, rigid stomach is incapable of expanding to accommodate ingested food.
Although scirrhous carcinomas are often manifested by relatively marked gastric narrowing, some tumors cause only mild loss of distensibility. Instead, these lesions may be recognized on double-contrast studies primarily by distortion of the normal surface pattern of the stomach with mucosal nodularity, spiculation, ulceration, or thickened, irregular folds (see Fig. 32-13 ). Thus, some lesions are likely to be missed if the radiologist relies too heavily on gastric narrowing as the major criterion for diagnosing these tumors.
Carcinoma of the Cardia
Tumors arising at the cardia are notoriously difficult to detect on conventional single-contrast barium studies. Because the overlying rib cage precludes manual palpation or compression of the fundus, even large lesions at the cardia may be obscured by crowded folds or relatively opaque barium, which prevents adequate visualization of this region. With double-contrast technique, however, it is possible to evaluate the normal anatomic landmarks at the cardia and surrounding gastric mucosa for signs of malignancy. As a result, double-contrast barium studies may detect lesions at the cardia that are missed on conventional single-contrast examinations.
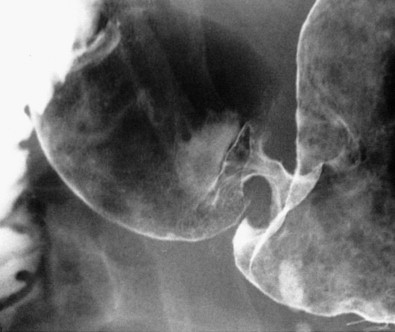
When viewed en face, the normal cardia often appears on double-contrast studies as a circular elevation containing four or five stellate folds that radiate to a central point at the gastroesophageal junction, also known as the cardiac rosette (see Chapter 26 ). Some lesions at the cardia may be recognized only by subtle nodularity, mass effect, or ulceration in this region with distortion, effacement, or obliteration of the rosette ( Fig. 32-14 ). Enlargement or lobulation of the surrounding elevation should also suggest a neoplastic lesion. Finally, an abnormal protrusion at the cardia should persist when additional barium is swallowed, whereas an apparent abnormality at the cardia must be an artifact if it vanishes as the lower esophageal sphincter relaxes and the cardia opens.
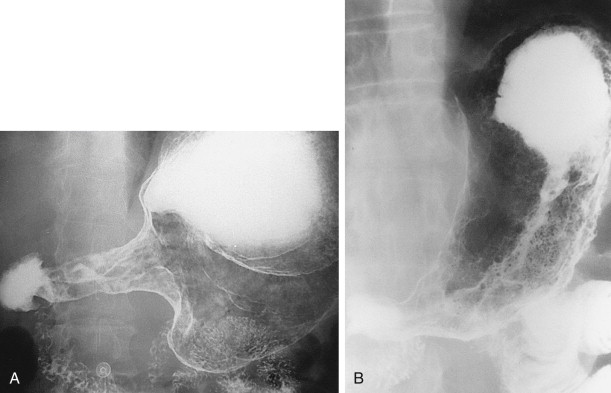
Advanced carcinomas of the gastric cardia or fundus may be polypoid or infiltrating lesions. Polypoid tumors usually appear as lobulated intraluminal masses in the gastric fundus, often containing irregular areas of ulceration. In contrast, infiltrating lesions are manifested by thickened, nodular folds and decreased distensibility of the fundus. Advanced tumors may completely encase the fundus, producing a linitis plastica appearance ( Fig. 32-15 ).
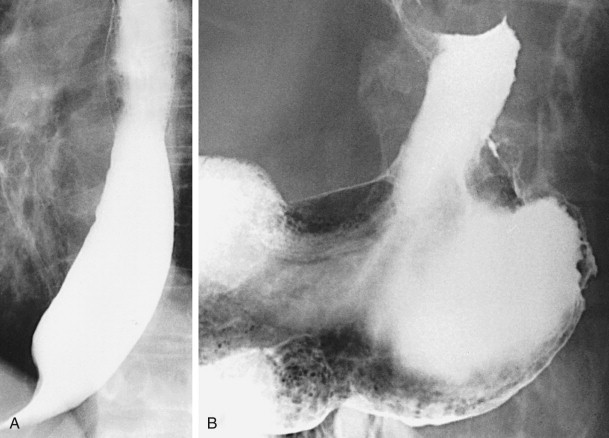
When an equivocal or suspicious lesion is detected in the region of the gastric cardia or fundus, endoscopy should be performed for a more definitive diagnosis. Nevertheless, radiographically demonstrated lesions at the cardia may occasionally be missed at endoscopy. The barium study should therefore be repeated, despite a negative endoscopic examination, if the initial barium study suggests a malignant lesion. Rarely, some patients with continuing radiologic evidence of malignancy may require surgery without preoperative histologic confirmation.
Secondary esophageal involvement by advanced lesions may be manifested on barium studies by a polypoid or fungating mass extending from the gastric fundus into the distal esophagus or by thickened folds or irregular narrowing of the distal esophagus without a discrete lesion (see Fig. 32-14B and C ). Esophageal involvement is usually confined to a 4- to 5-cm segment above the gastroesophageal junction but may occasionally extend as far proximally as the aortic arch. Submucosal spread of tumor can also result in secondary achalasia with tapered, beaklike narrowing of the distal esophagus at or just above the gastroesophageal junction (see Fig. 32-15A and Chapter 24 ). However, certain morphologic features such as asymmetry, abrupt transitions, and mucosal nodularity or ulceration should suggest an underlying malignancy. Secondary achalasia should also be suspected when the narrowed segment extends proximally a discrete distance from the gastroesophageal junction. In such cases, careful radiologic evaluation of the gastric fundus is essential to rule out a carcinoma of the cardia as the cause of these findings.
Computed Tomographic Findings
The widespread availability of multidetector CT (MDCT) scanners has improved the diagnosis of gastric carcinoma on CT because of its ability to create high-quality images in any conceivable view plane ( Fig. 32-16 ). Detection of these lesions requires optimal gastric distention to efface the overlying rugal folds and accentuate areas of asymmetry along the contour of the stomach. Some authors recommend oral administration of a neutral (water-attenuation) contrast agent, whereas others recommend the use of an oral effervescent agent (as is used for double-contrast barium studies of the stomach) to optimize gastric distention.