Chapter Outline
Retroperitoneal Anatomy: Spaces
Retroperitoneal Anatomy: Planes
Subperitoneal Spaces: Ligaments and Mesenteries
Peritoneal Spaces: Upper Abdomen
Peritoneal Spaces: Lower Abdomen and Pelvis
The peritoneum, subperitoneal space, and retroperitoneum are anatomic compartments frequently involved in pathologic processes that originate in the gastrointestinal tract. Because they represent the result of complex embryologic processes of organogenesis, rotation, folding, and fusion, the distribution of fluid collections resulting from such processes is often confusing. A working knowledge of the anatomic compartmentalization of disease spread in the abdomen is essential to the understanding of imaging findings in a variety of pathologic conditions.
This chapter focuses on the anatomy of the peritoneum, retroperitoneum, and subperitoneum as depicted by cross-sectional imaging methods. In normal individuals, the boundaries of these anatomic regions are defined poorly or not at all. When pathologic processes produce intra-abdominal fluid collections, the patterns of fluid distribution are directly determined by their relationships. The patterns of disease spread within the abdomen can be logically predicted from an understanding of the folds of mesenchymal tissue that envelop the gut and the gut-derived solid viscera: the mesenteries. Most illustrations in this chapter feature the axial sections familiar to all practitioners of computed tomography (CT) and magnetic resonance imaging (MRI). In selected examples, however, the anatomic relationships are better displayed by coronal, sagittal, or volume rendered images.
Embryology
Embryology is the key to understanding all of the concepts presented in this chapter. This section provides an overview of the embryogenesis of the retroperitoneum and the mesenteries that form ligamentous attachments in the adult. Some details are addressed in the sections on retroperitoneum and subperitoneum that follow.
Upper Abdominal Embryogenesis
The Gut and Its Derivatives
At about 3 weeks of fetal life, the lateral body plates (the mesodermal tissue forming the external fetal surface) of the fetus curl up to enclose a small portion of the celomic cavity to form an intraembryonic celomic cavity ( Fig. 108-1A ). Near the umbilicus, a portion of the yolk sac, which gives rise to the endodermal structures (the gut and all gut-derived solid organs), is enclosed and lined on all sides by fetal mesoderm. At this stage, there are well-demarcated right and left peritoneal spaces, divided by the mesenchyme suspending the gut from the ventral body wall (i.e., ventral mesentery), the gut and its surrounding mesenchyme, and the mesenchyme suspending the gut from the dorsal body wall (i.e., dorsal mesentery). Maternal blood from the umbilical vein courses through the ventral mesentery to supply the fetus, and blood from the now paired dorsal aortae establish vascular channels to the gut and developing viscera through the dorsal mesentery.

At about 4 weeks of fetal development, this symmetric arrangement is distorted. The liver begins to develop in the middle of the ventral mesentery, and it grows rapidly in all directions, bulging the contour of the right and left ventral peritoneal space and extending superiorly inside the ventral mesentery until it encounters the septum transversum (i.e., fetal diaphragm), which is growing from the ventral to the dorsal wall of the fetus to separate the thoracic from the abdominal cavity. The broad, roughly oval line of contact between the ventral mesentery and the septum transversum is not lined by mesothelium, and it becomes the bare area of the liver in the adult ( Fig. 108-1B, C ).
At the same time, the spleen and pancreas are beginning to grow within the leaves of the dorsal mesogastrium ( Fig. 108-1D ). The spleen generates just dorsal to the stomach, whereas the dorsal pancreas arises behind the duodenum. Part of the pancreas (i.e., the head and uncinate process in the adult) arises from a nest of cells within the ventral mesentery that ultimately rotates to join the dorsal pancreas later in fetal life. The all-enveloping mesenchyme continues to connect all of these developing organs with the body wall and the gut, and it persists into adult life as the abdominal ligaments ( Fig. 108-1E ). The ventral part of the ventral mesentery, connecting the liver to the anterior body wall, becomes the falciform ligament, through which courses the umbilical vein (and its fibrous remnant, the ligamentum teres, in the adult). The dorsal part of the ventral mesentery becomes the gastrohepatic and hepatoduodenal ligaments, which together compose the lesser omentum. The gastrohepatic ligament stretches between the lesser curvature of the stomach and the fissure for the ligamentum venosum. It contains the left gastric artery and vein and the lymph node group that drains the lesser curvature. The hepatoduodenal ligament, the caudal extension of the lesser omentum, contains the portal vein, hepatic artery, common bile duct, and hepatic lymph node chain. The most inferior edge of the lesser omentum forms the roof of the foramen of Winslow.
The ventral part of the dorsal mesentery, connecting the stomach to the spleen, becomes the gastrosplenic ligament, identified by the short gastric arteries and veins that course within it ( Fig. 108-1F ). In the adult, the gastrosplenic ligament also forms the entirety of the greater omentum (i.e., gastrocolic ligament) and the cephalad portion of the transverse mesocolon (discussed later). These fat-filled structures are readily identified by their contained vessels, the gastroepiploic in the greater omentum and the middle colic in the transverse mesocolon. The dorsal part of the dorsal mesentery becomes the splenorenal (lienorenal) ligament, which contains the splenic vessels and connects the spleen with the tail of the pancreas. This latter mesenchyme fuses almost completely with the anterior renal fascia to become part of the retroperitoneum.
During the fifth and sixth weeks of fetal life, the liver continues to grow rapidly and rotates into the right peritoneal space. Concurrently, the stomach undergoes a 90-degree clockwise rotation, such that its left side becomes ventral and its right side dorsal. This rotation pulls the dorsal mesogastrium to the left, allowing room for the right peritoneal space to expand behind the stomach and in front of the pancreas.
This part of the right peritoneal space becomes the lesser sac (i.e., omental bursa). The inferior margin of the gastrosplenic ligament elongates in a caudal direction to form a long inferior sinus. Later in fetal life, the redundant apposed layers of this part of the gastrosplenic ligament fuse to form the greater omentum ( Fig. 108-2 ). During this period (roughly week 6 of fetal development), the midgut has lengthened to the extent that its midportion projects into the umbilical cord (i.e., physiologic herniation). During the 10th week, it returns to the abdomen. The cecum ultimately undergoes a 180-degree rotation (as viewed from above) to arrive at its normal location in the right lower quadrant. The ascending and descending colonic segments undergo further rotation such that their mesenteries fuse to the anterior renal fascia on each side ( Fig. 108-3 ). On the right, the ascending colon rotates 90 degrees counterclockwise (as viewed from below) and normally becomes a fixed part of the retroperitoneum. On the left, the descending colon rotates 90 degrees clockwise (as viewed from below) to become a portion of the retroperitoneum. The transverse colon and its mesentery span the fetus from right to left, with a mesenteric surface facing ventrally. The posterior part of the gastrosplenic ligament then fuses with this surface to form the adult transverse mesocolon.

Lower Abdominal and Pelvic Embryogenesis
Rectum, Urogenital Sinus, and Kidneys
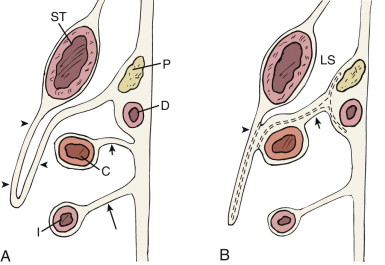
The rectum and urogenital sinus initially develop as part of one structure, the cloaca, the distal part of the hindgut. As the fetus develops, the urorectal fold, a peritoneal sinus, divides the anterior urinary system from the posterior gut ( Fig. 108-4A ). In the adult, the caudal part of the urorectal fold fuses to become Denonvilliers’ fascia, but a sulcus of peritoneum persists in males and females, becoming the rectovesical pouch (i.e., pouch of Douglas). The rectum and perirectal fat are enveloped by an important fascial plane, the mesorectal fascia.

The anterior (urinary tract) system has several connections that determine adult anatomic landmarks. Superiorly, the urogenital sinus is connected to the umbilical stalk through the allantois, which undergoes fibrous degeneration to become the urachus in both sexes. Posteriorly, the midportion of the urogenital sinus (which becomes the urinary bladder) receives the metanephric duct, or ureter. The inferior part of the urogenital sinus receives the paramesonephric duct and the mesonephric duct; further development of the sinus and the two ducts it receives depends on gender differentiation. In the female embryo, the inferior parts of the paramesonephric ducts fuse to become the uterus and upper vagina, and the part closest to the gonad becomes the fallopian tube. The mesonephric duct almost completely degenerates. The inferior aspect of the urogenital sinus becomes the urethra, lower vagina, and vestibule, with associated Bartholin’s glands ( Fig. 108-4B ).
In the male embryo, the paramesonephric ducts degenerate. The portion of the mesonephric duct closest to the gonads becomes the ductus deferens, and the part closest to the urogenital sinus develops into the seminal vesicles and ejaculatory duct. Meanwhile, the urogenital sinus becomes the prostate, urethra, and associated glands ( Fig. 108-4C ). The remnant of this embryologic derivation is a fascial plane (i.e., umbilicovesical fascia) that envelops the extraperitoneal portion of the bladder and is continuous with the structures that were embryologically in communication with the bladder surface. These structures include the urachus (i.e., median umbilical ligament), the obliterated umbilical arteries (i.e., medial umbilical ligaments), and, in males, the ductus deferens, seminal vesicles, and prostate. This fascia also covers the medial aspect of the ureters as they insert into the bladder base (see Fig. 108-15 ).
Retroperitoneal Anatomy: Spaces
The embryology previously discussed provides a rationale for the laminar nature of the retroperitoneum ( Fig. 108-5 ). We consider four layers, proceeding from the outermost portion of the extraperitoneum (i.e., body wall and great vessels) to the innermost (i.e., anterior pararenal space). The basic concept to be stressed is that pathologic fluid collections rapidly fill their space of origin and then extend into some nearby expandable plane. If the origin of the fluid collection is within what was embryologically a mesentery (e.g., pancreatitis within the dorsal mesentery), a common mode of extension is within the ligament to which it is attached (e.g., the gastrosplenic ligament). This method of extension is known as subperitoneal spread, as shown in Figure 108-5A . Alternatively, fluid collections may progress between the layers that make up the retroperitoneal lamina (e.g., between the anterior pararenal space and the perirenal space). This is known as interfascial spread, and it provides potential communication between the upper abdomen and the pelvis ( Fig. 108-5B, C ). The remainder of this section provides clinical images of each form of spread, including examples of fluid and aggressive cellular collections.

Great Vessels
The aorta and inferior vena cava course through an incompletely marginated space in front of the vertebrae and anterior psoas fascia and posterior to the root of the small intestinal mesentery. An incomplete lateral boundary is formed by the medial aspect of the anterior and posterior renal fasciae, but this is pierced by the renal artery and vein. Superiorly, the great vessel space is continuous with the middle mediastinum; inferiorly, it extends between the abdominal portions of the ureters. Processes that begin in this space can extend easily into the perirenal fat or into the planes anterior and posterior to the perirenal space ( Fig. 108-6 ). Hemorrhage from ruptured aortic aneurysms is particularly likely to enter the posterior interfascial plane. Other processes, such as retroperitoneal fibrosis, tend to be restricted to the great vessel space, encompassing the aorta and inferior vena cava and extending to involve both ureters in their abdominal course.

Transversalis Fascia
The transversalis fascia is the expandable plane that lines the inner surface of the entire abdominal wall. It forms the outermost layer of the retroperitoneum. It is continuous with the diaphragmatic fascia superiorly and with the deep pelvic fascia inferiorly, so that it provides a natural pathway for fluid collections to spread throughout the abdominal cavity.
Posterior Fat Pads
There are two fat pads that provide a cushion for the kidneys; they are positioned in the posterolateral portion of the abdomen and extend into the upper portion of the pelvis. The larger of the two is a crescentic fat collection, the anterior part of which lies lateral to the ascending and descending colon; the posterior part lies on the posterior surface of the perirenal fat. This fat has been called the posterior pararenal space. Cutaneous nerves pass through this fat, but it contains no organs and is rarely the primary site of pathologic processes. The smaller fat pad lies just anterior to the transversalis fascia near the quadratus lumborum muscle. Between the two fat pads is a cleft, through which fluid may pass from the deep retroperitoneum outward to the transversalis fascia. Hernias containing fat or bowel can pass through this same cleft, which is a part of the inferior lumbar triangle (i.e., Petit’s triangle). It is common for severe pancreatitis to spread by this route, producing inflammation or hemorrhage, or both, in the ipsilateral flank; this is the rationale for the Grey Turner sign of hemorrhagic pancreatitis ( Fig. 108-7 ).
Perirenal Space
There has been considerable interest in delineating the precise anatomy of the perirenal space and its enveloping fascia, and there is no universal agreement about its exact boundaries—whether its inferior part is open or closed to the pelvis and whether the spaces communicate across the midline. In our view, rapidly expanding retroperitoneal fluid collections extend into the interfascial planes, where they can freely spread into the pelvic extraperitoneum and across the midline (examples are discussed later).
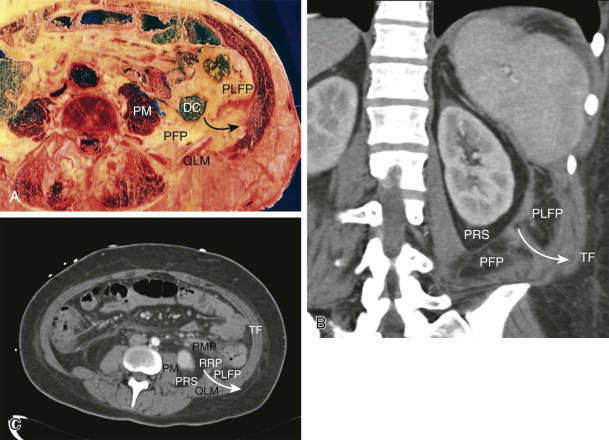
The embryology of the kidneys provides the explanation for the unusual appearance of the perirenal space. As the kidneys ascend from their pelvic origins, they are sheathed by a long, tapered cone of fat within the anterior and posterior renal fascia. This space is thin at the diaphragm, typically most voluminous posterior to the lower renal pole, and thin again as it extends inferiorly toward the pelvis. Fluid collections, such as urinomas or localized hematomas, often gravitate toward the posteroinferior portion of the perirenal fat, which typically has the greatest volume. The lateral boundary of the perirenal space abuts the posterolateral fat pad, and its fusion with that space creates an expandable plane that extends behind and lateral to the kidney, the retrorenal plane. The laminar nature of this fusion plane was described by Raptopoulos and colleagues and conforms to clinical observations. The anterior boundary of the perirenal space abuts the anterior pararenal space and produces a similar laminar plane (i.e., retromesenteric plane) that also collects and distributes retroperitoneal effusions ( Fig. 108-8 ).

Each perirenal space contains the kidney, adrenal gland, proximal ureter, renal artery, and renal vein. The fascial lining of the perirenal space is incomplete where the renal vessels pass through it, and the entire medial boundary of the space is poorly defined. The study by Kunin established that there are well-structured pores within the perirenal fat, some of which course between one surface of the kidney and another (i.e., renorenal septa) and some of which communicate between the renal surface and the perirenal fascia (i.e., renofascial septa). The latter are responsible for the rapid egress of effusions (often urinomas ) in the perirenal space into the retromesenteric or retrorenal plane ( Fig. 108-9 ) and for involvement of the renal surface with effusions of extrarenal origin, such as pancreatitis.

Anterior Pararenal Space
The anterior pararenal space is entirely composed of visceral structures and their mesenteries of origin. Medially, the components consist of the pancreas (with remnants of the ventral mesentery surrounding the head and uncinate process and the dorsal mesentery supporting the neck, body, and tail) and the duodenum. These structures fuse posteriorly with the perirenal space, typically at the level of the renal hila. In the midline, the fusion plane passes between the great vessel space and the root of the intestinal mesentery. This part of the retromesenteric plane provides communication between the left and right retroperitoneum, and it is the only anatomic site where a passage exists across the midline (see Fig. 108-12C ).
The lateral aspect of the pararenal space is formed by the ascending and descending colon and their attached dorsal mesenteries. These mesenteries fuse to the anterior aspect of the perirenal fat just lateral to the fusion of the duodenum, and they create a single retromesenteric plane on each side. Laterally, they fuse to the posterolateral fat pads to form the lateroconal plane ( Fig. 108-10 ).