Cardiac Anatomy, Physiolgy, and Imaging Modalities
David K. Shelton
Imaging Methods
Thorough knowledge of cardiac anatomy and physiology is important as a basis for cardiac imaging. Comprehensive knowledge of cardiac imaging also requires consideration of virtually all the available imaging modalities. Chest radiography provides the initial evaluation of most cardiac patients. A barium esophagram can provide additional information because of the close relationship of the esophagus to cardiac structures. Fluoroscopy increases the detectability of coronary and valvular calcification as well as provides dynamic and positional information. Transthoracic echocardiography, including pulse wave and color flow Doppler, and transesophageal echocardiography provide additional detailed imaging of internal cardiac anatomy and function. Nuclear cardiology, PET, and pharmacologic testing provide key functional, perfusion, and physiologic information. Cardiac and coronary angiography, although invasive, can provide detailed anatomic information that can lead directly to interventional or surgical therapy. CT, MDCT, CT angiography (CTA), and ultrafast CT with the use of IV iodinated contrast material are capable of providing critical information, particularly for pericardial or intracardiac disease. Recent technological advances in the latter also allow detection of premature coronary calcification, which may have prognostic implications. MR adds three-dimensional (3D) tomographic and motion studies of the myocardium, valves, and chambers without using ionizing radiation or intravascular contrast. Cardiac imaging requires familiarity with all imaging techniques and their associated physics, 3D cardiac anatomy, cardiac physiology, and cardiac disease processes.
Anatomy
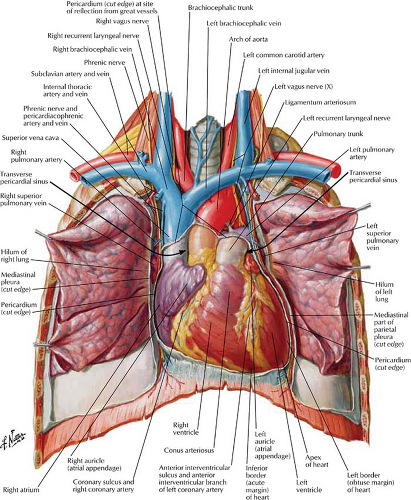
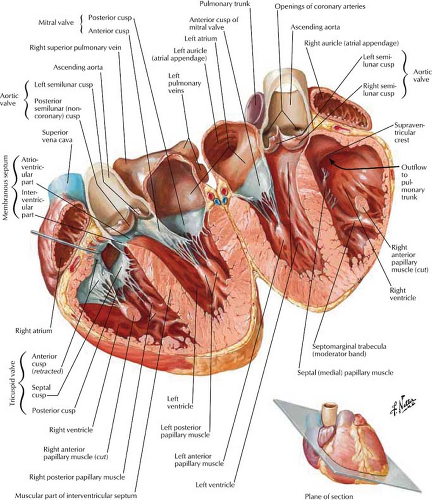
The four-chambered heart lies primarily in the anterior left hemithorax with the LV lying on the left hemidiaphragm (Figs. 21.1, 21.2). The RA extends to the right of the midline as it receives systemic blood from the superior vena cava (SVC), inferior vena cava (IVC), and coronary sinus. The RA and RV lie primarily anterior to the planes of the LA and LV. The RV is the most anterior chamber and abuts the sternum (Fig. 21.3). The LA is subcarinal and midline in the thorax, being supplied by the right and left superior and inferior pulmonary veins.
Frontal Projection. The right border of the cardiac silhouette is formed primarily by the RA, with the SVC entering superiorly and the IVC often seen at its lower margin (Figs. 21.1, 21.3). The left border of the heart is created primarily by the LV and LA appendage. The PA, aortopulmonary window, and aortic knob extend superiorly.
Lateral Projection. The RV is border forming anteriorly adjacent to the sternum, with its outflow tract extending superiorly and posteriorly (Fig. 21.2). The LA is border forming in the high posterior, subcarinal region. The LV is border forming inferiorly and posteriorly.
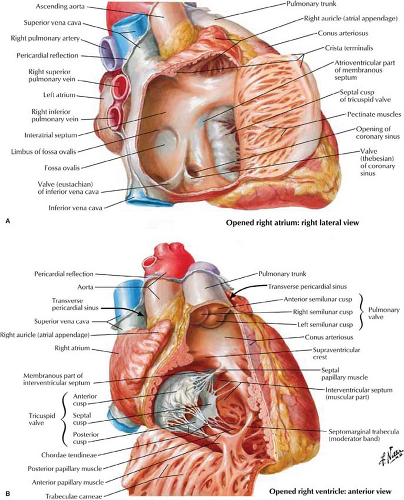
Right Atrium. The RA is divided into two portions. The smooth posterior wall develops from the sinus venosus, with the attached SVC and IVC in continuity posteriorly (Fig. 21.4). The trabeculated anterior wall is derived from the embryonic RA. The RA appendage extends superiorly and medially from the SVC opening. The crista terminalis is a muscular ridge that runs from the mouth of the SVC and fades inferiorly to the mouth of the IVC. It divides the two portions of the atrium
and corresponds to an external sulcus terminalis. The medial or posterior wall of the RA is the interatrial septum, which contains a smooth, central dimpled area called the fossa ovalis. Inflow from the SVC, IVC, and coronary sinus enters the smooth posterior portion of the RA. The SVC has a free opening, whereas the IVC is partially guarded by a thin eustachian valve, which is occasionally absent or perforated (network of Chiari). The large draining coronary vein or coronary sinus enters the RA anterior and medial to the IVC. Its opening is guarded by the thebesian valve between the orifice of the IVC and the tricuspid valve.
and corresponds to an external sulcus terminalis. The medial or posterior wall of the RA is the interatrial septum, which contains a smooth, central dimpled area called the fossa ovalis. Inflow from the SVC, IVC, and coronary sinus enters the smooth posterior portion of the RA. The SVC has a free opening, whereas the IVC is partially guarded by a thin eustachian valve, which is occasionally absent or perforated (network of Chiari). The large draining coronary vein or coronary sinus enters the RA anterior and medial to the IVC. Its opening is guarded by the thebesian valve between the orifice of the IVC and the tricuspid valve.
Right Ventricle. The RV (Figs. 21.4, 21.5) lies anterior to the left ventricular outflow tract and wraps around it and to the left. The right ventricular outflow is directed superiorly, posteriorly, and to the left. The RV is divided into a posterior or inferior portion (inflow or sinus portion), which is heavily trabeculated, and a less trabeculated anterior or superior portion (outflow tract or pulmonary conus). The two portions of the RV are divided by the crista supraventricularis, which is a muscular ridge with a septal band called the moderator band. This band is present in more than 40% of patients, connects the interventricular septum to the anterior papillary muscle, and contains the right bundle branch. The infundibulum (conus arteriosus) is the smooth cephalic portion of the RV that leads to the pulmonary trunk.
Pulmonary Arteries. The muscular pulmonary conus extends to the semilunar, tricuspid pulmonary valve, with the pulmonary trunk extending superiorly and to the left. The left PA extends posteriorly as a continuation of the main PA, coursing over the top of the left main stem bronchus, then descending posteriorly. The right PA extends horizontally to the right, bifurcates within the pericardial sac, and exits the right hilum as the truncus anterior and interlobar arteries. The left main stem bronchus is hyparterial, meaning that it lies below the PA. The right bronchus is eparterial, meaning that it lies next to the right PA.
The ligamentum arteriosum arises from the superior, proximal left PA and crosses through the aorticopulmonary window to the floor of the aorta. The ligamentum arteriosum is the remnant of the ductus arteriosus, which closes functionally in the first 24 hours and closes anatomically by 10 days. Desaturated blood from the right heart circulates through the lungs and returns as oxygenated blood through the right and left superior and inferior pulmonary veins into the LA.
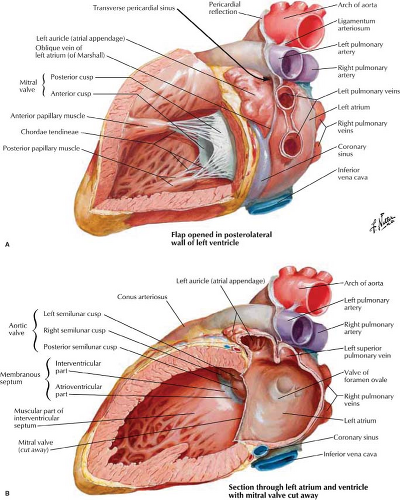
Left Atrium. The LA is the highest and most posterior chamber (Fig. 21.6). Its smooth walls are nestled between the right and left bronchi, and its posterior wall abuts the anterior wall of the esophagus. The left atrial appendage is a small pouch that projects superiorly and to the left and is smoother and longer than the right atrial appendage. The left atrial appendage extends anterior to the left superior pulmonary veins and is readily seen on MR and CT scans. The foramen ovale within the interatrial septum remains nominally patent in up to 25% of adults. Its inferior margin is a remnant of the septum primum and may be somewhat scalloped. The mitral valve is located anterior and inferior to the body of the LA, with the mitral valve leaflets extending into the LV.
Left Ventricle. The mitral valve is the conduit for blood flow from the LA to the LV and is in the high posterior “valve plane” of the LV (Figs. 21.5, 21.6). The anterior or septal leaflet of the mitral valve lies near the interventricular septum and extends to the posterior (noncoronary) cusp of the aortic valve. The smaller posterior mitral leaflet lies posteriorly and to the left. The chordae tendineae are strong fibrous cords that extend from the mitral leaflets to the papillary muscles of the LV. The inflow portion of the LV is posterior to the anterior leaflet of the mitral valve. The outflow portion of the LV is anterior and superior to the anterior mitral leaflet. The interventricular septum has a high membranous portion that is contiguous with
the aortic root. The more muscular inferior portion of the septum extends to the left ventricular apex. The esophagus passes immediately posterior and is in contact with the muscular wall of the LV.
the aortic root. The more muscular inferior portion of the septum extends to the left ventricular apex. The esophagus passes immediately posterior and is in contact with the muscular wall of the LV.
Aorta. The outflow tract of the LV leads into the aortic root through the aortic valve which is composed of right, left, and posterior (noncoronary) cusps. The sinuses of Valsalva are the reservoirs created by the closure of the aortic valve and from which the right and left coronary arteries arise. The posterior wall of the aorta is continuous with the anterior leaflet of the mitral valve and more superiorly abuts the anterior wall of the LA. The anterior wall of the aorta is continuous with the interventricular
septum. After coursing superiorly and then to the left, the aorta gives off the right innominate artery, left common carotid artery, and left subclavian artery. The aortic arch is the transverse portion of the aorta that abuts the left wall of the trachea, causing a characteristic indentation.
septum. After coursing superiorly and then to the left, the aorta gives off the right innominate artery, left common carotid artery, and left subclavian artery. The aortic arch is the transverse portion of the aorta that abuts the left wall of the trachea, causing a characteristic indentation.
Conduction System. The sinoatrial node consists of specialized neuromuscular tissue that measures approximately 5 to 20 mm and is located on the anterior endocardial surface of the RA just above the SVC and right atrial appendage junction, near the crista terminalis. Electrical propagation spreads to both atria via Purkinje-like fibers and is recorded as the P wave on an electrocardiogram. The atrioventricular node is a 2 × 5 mm region of neuromuscular tissue on the endocardial surface, along the right side of the interatrial septum, just inferior to the ostium of the coronary sinus. The impulse is collected and delayed approximately 0.7 seconds in the atrioventricular node before passing into the bundle of His. The bundle of His is a 20-mm-long tract which extends down the right side of the membranous interventricular septum. The bundle of His bifurcates into a right and left bundle before arborizing through the two ventricles via the Purkinje system. The interventricular septum activates from superior to inferior, with the anterior or septal RV being the first to activate and the posterior or basal LV being the last to activate. This information is particularly useful when evaluating phase analysis or phase propagation in gated cardiac scintigraphy.
Cardiac Catheterization
Left-sided catheterization is normally accomplished via arterial puncture in the femoral or brachial artery (Fig. 21.7). It is typically used for aortography, coronary and coronary bypass graft angiography, ventriculography, and evaluation for patent ductus arteriosus. Right-sided catheterization is typically accomplished by venous puncture in the femoral or brachiocephalic vein (Fig. 21.8). It is used for pulmonary angiography, catheterization of the RA and RV, or evaluation of shunt lesions such as an atrial septal defect.
Important considerations include determination of the catheter course to help diagnose atrial septal defects (ASDs), ventricular septal defects (VSDs), patent ductus arteriosus, or persistent left SVC. During catheterization, oxygen saturation percentages are commonly determined, along with pressure measurements and pressure gradients (Table 21.1). Contrast is injected to demonstrate additional details of anatomy, as well as to evaluate for valvular lesions, chamber size, ventricular function, and wall motion.
Right atrial pressures are normally 2 to 5 mm Hg and oxygen saturation is 65% to 75%. Elevated right atrial pressures are seen with right heart failure, decreased compliance, and tricuspid valve disease. A 7% or greater increase in saturation from the IVC to the RA is considered evidence of a left-to-right shunt (ASD).
Right ventricular pressures are typically 25 systolic and 0 to 5 diastolic mm Hg. Elevated systolic pressures are seen with
pulmonary hypertension, pulmonic valve stenosis, and congenital heart lesions such as transposition and truncus arteriosus. Diastolic pressures increase with right heart failure. Saturations should be nearly the same as right atrial saturations. A 5% increase in saturation from RA to RV suggests a VSD.
pulmonary hypertension, pulmonic valve stenosis, and congenital heart lesions such as transposition and truncus arteriosus. Diastolic pressures increase with right heart failure. Saturations should be nearly the same as right atrial saturations. A 5% increase in saturation from RA to RV suggests a VSD.
Table 21.1 Normal Values for Cardiac Catheterization | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Table 21.2 Average Physiologic Data for Cardiac Chambers | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pulmonary arterial pressures are normally 25 systolic and 10 diastolic mm Hg, with a mean PA pressure of 15 mm Hg. A significant pressure gradient (>10 mm Hg) across the valve implies pulmonic valve stenosis. Increased pressures are seen with shunt lesions, pulmonary vascular disease, and pulmonary venous obstruction. Pulmonary arterial saturation should be approximately the same as right ventricular saturation, with a 3% difference considered significant for a shunt lesion.
Pulmonary capillary wedge pressure is typically 2 to 8 mm Hg and approximates the left atrial pressure unless there is evidence of pulmonary venous obstruction. Elevations in the left atrial or wedge pressure are usually seen with mitral stenosis and left-sided congestive heart failure. Normal left atrial saturation is approximately 94%, and a decrease greater than 5% implies a right-to-left shunt.
Left ventricular pressures are normally approximately 120 systolic and 0 to 5 diastolic mm Hg. Decreased systolic pressures are seen with shock and congestive heart failure. Elevated systolic pressures imply systemic hypertension or outlet obstruction. Increased diastolic pressure is seen with congestive heart failure. Decreased saturation at the left ventricular level would imply a right-to-left shunt. Aortic pressure is normally approximately 120 systolic and 80 diastolic, with a mean pressure of 70 to 100 mm Hg.
With each systolic contraction, the average stroke volume of each ventricle is 70 mL of blood (Table 21.2). End diastolic volume is normally 125 to 150 mL for the LV and 165 mL for the RV. A normal cardiac output is 4 to 5 L/min, with a normal cardiac index of 2.8 to 4.0 L/min/m2 of body surface area. The normal ejection fraction is 50% to 75% for the LV and 45% to 55% for the RV. Typical end diastolic volumes are 57 mL for the RA and 50 mL for the LA. Coronary blood flow averages approximately 224 mL/min and increases up to sixfold during exercise.
Aortic Valve. The normal aortic valve orifice is 3 cm2. Symptoms result from aortic stenosis usually when either the orifice is less than 0.7 cm2 or when it is less than 1.5 cm2 if there is aortic stenosis and insufficiency. Mild stenosis is indicated by a pressure gradient across the aortic valve greater than 25 mm Hg, moderate stenosis by a gradient greater than 40 to 50 mm Hg, and severe stenosis by a gradient exceeding 80 mm Hg.
Mitral Valve. The mitral valve orifice usually measures 4 to 6 cm2. Mild mitral stenosis occurs with an orifice less than 1.5 cm2, moderate mitral stenosis at less than 1.0 cm2, and severe mitral stenosis at less than 0.5 cm2.
Pulmonic stenosis is considered significant if the right ventricular systolic pressure exceeds 70 mm Hg.
PA hypertension is defined as a mean PA pressure of more than 25 mm Hg.
Chest Radiography
The chest radiograph remains a mainstay for imaging of the heart and lungs. There are many approaches to reading the radiograph. Although most radiologists initiate the process with “global perception,” it is important to develop a checklist scan technique. This discussion concerns adult posteroanterior and lateral radiographs.
Cardiac Silhouette
Size. The cardiothoracic ratio should not exceed 0.5 on a 72-in erect posteroanterior radiograph or 0.6 on a portable or anteroposterior (AP) examination. Other factors should be considered, such as fat pads and pectus deformity.
Shape. Various contour effects can be clues to underlying disease. “Water bottle” configuration occurs with pericardial effusion or generalized cardiomyopathy. Left ventricular or “Shmoo” configuration (after Al Capp’s Shmoo) describes lengthening and rounding of the left heart border with a downward extension of the apex resulting from left ventricular enlargement. “Hypertrophy” configuration describes increased convexity of the left heart border and apex. Right ventricular hypertrophy and enlargement tends to lift the apex and create a more horizontal vector to the cardiac axis. Hypertrophy of either ventricle usually causes little enlargement of the silhouette unless dilatation is also present. Hypertrophy typically results from increased afterload, whereas dilatation occurs with failure or diastolic overload. “Straightening” of the left heart border is seen with rheumatic heart disease and mitral stenosis.
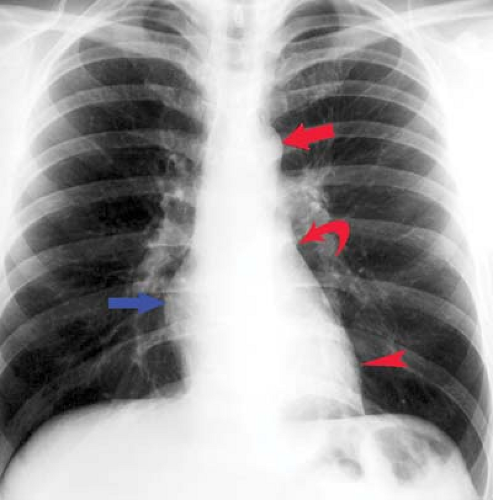
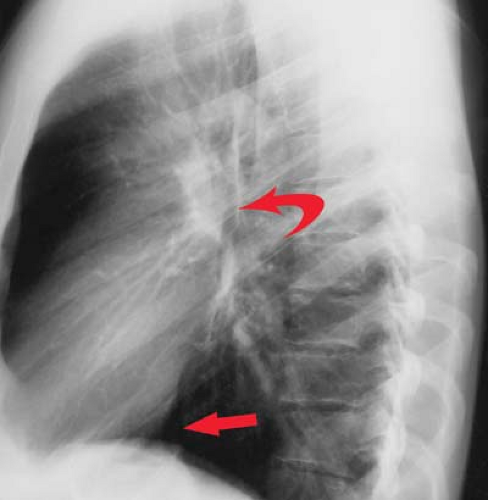
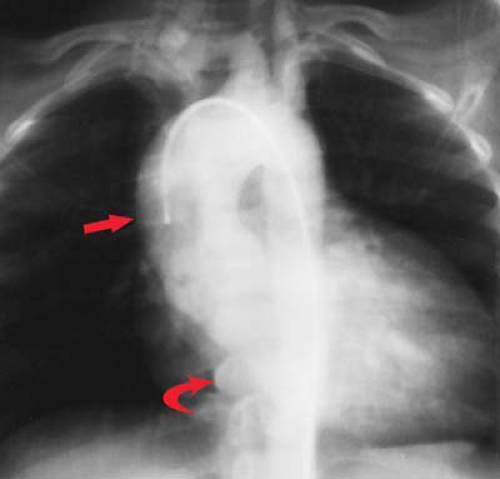
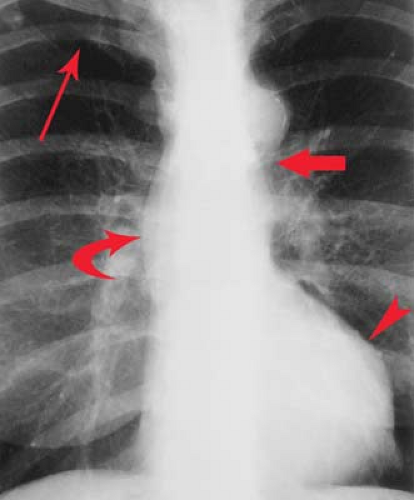
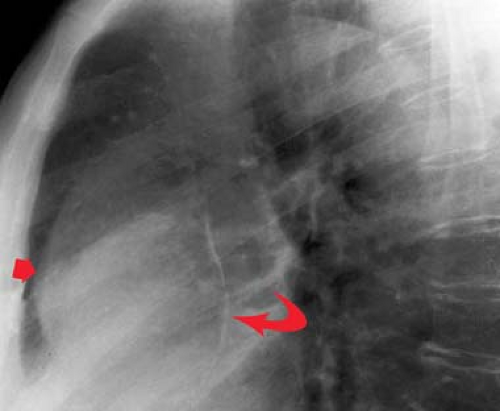
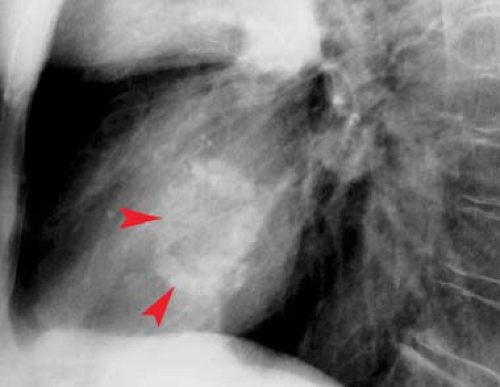
“Moguls of the Heart.” Skiing the moguls of the heart refers to the left mediastinal outline beginning at the aortic knob. A prominent knob is a clue to ectasia, aneurysm, or hypertension. Notching or “figure 3” sign of the aorta suggests coarctation (Fig. 21.9). The second mogul is the main PA segment. Excessive convexity is seen with poststenotic dilatation, chronic obstructive pulmonary disease, PA hypertension, left-to-right shunts, and pericardial defects. Severe concavity suggests right-to-left shunts. The third mogul is a prominent left atrial appendage that in 90% of cases indicates prior rheumatic carditis (Fig. 21.10). It is not usually seen with other causes of left atrial enlargement. The fourth mogul is a bulge just above the cardiophrenic angle, seen with infarction or ventricular aneurysm. A fifth bulge at the cardiophrenic angle is caused by pericardial cysts, prominent fat pads, or adenopathy.
Chamber Enlargement
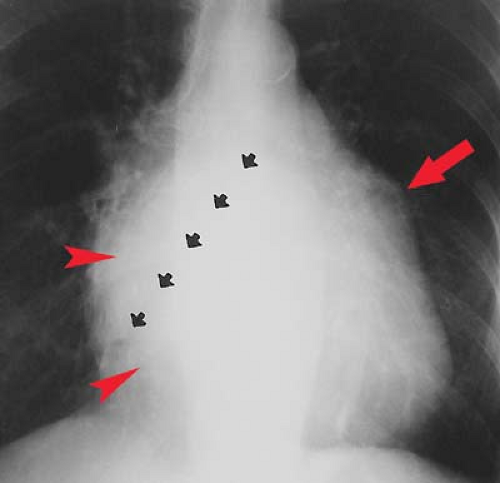
Left atrial enlargement is best confirmed by measuring the distance from the midinferior border of the left main stem bronchus to the right lateral border of the left atrial density (see Fig. 21.10). This distance is less than 7 cm in 90% of normal patients and is greater than 7 cm in 90% of patients with left atrial enlargement, as proven by echocardiography. This measurement can be approximated by placing one’s right fifth finger under the left bronchus and while keeping the fingers closed, determining whether the LA is seen beyond one’s four fingertips; if so, the LA is enlarged. Less sensitive signs of left atrial enlargement include splaying of the carinal angle, uplifting of the left main stem bronchus, and prominence of the left atrial appendage. On occasion, the enlarged LA will displace the descending aorta to the left. Massive left atrial enlargement can result in the LA becoming border forming on the right side, so-called “atrial escape.” On lateral views, an enlarged LA will displace the left bronchus posteriorly, with the bronchi creating right and left legs for the “walking man sign.” An enlarged LA also impresses against the esophagus.
Right atrial enlargement is more difficult to define on chest radiographs than left atrial enlargement, but fortunately, it is less common. Clues include a prominent atrial bulge too far to the right of the spine (more than 5.5 cm from the midline on a well-positioned posteroanterior radiograph). Another sign is elongation of the right atrial convexity to exceed 50% of the mediastinal or cardiovascular shadow. Right atrial enlargement usually accompanies right ventricular enlargement.
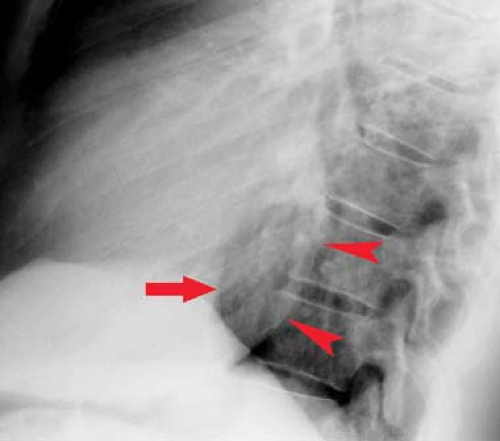
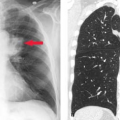
Left ventricular enlargement creates on the posteroanterior view an elongated left heart border with the apex pointing downward. Prominent rounding of the inferior left heart border is also seen (Fig. 21.11). The lateral view shows an enlarged LV extending behind the esophagus. The Hoffman–Rigler sign for left ventricular enlargement exists when the LV extends more than 1.8 cm posterior to the posterior border of the IVC at a level 2 cm cephalad to the intersection of the LV and IVC (Fig. 21.12). This sign requires a true lateral radiograph and can be false-positive if the lateral view is obliqued or there is volume loss in either lower lobe. This sign can be quickly applied by using one of the “2-cm fingertips” for a quick check without a ruler.
Right ventricular enlargement is not as easily detected as left-sided enlargement. If the heart is enlarged and Rigler sign does not show left ventricular enlargement, then consider right-sided enlargement. If the RV fills too much of the retrosternal clear space or “climbs” more than one-third of the sternal
length, then right ventricular enlargement is likely. Indirect signs such as enlargement of the pulmonary outflow tract or hilar arteries add confidence.
length, then right ventricular enlargement is likely. Indirect signs such as enlargement of the pulmonary outflow tract or hilar arteries add confidence.
Abnormal Mediastinal Contours
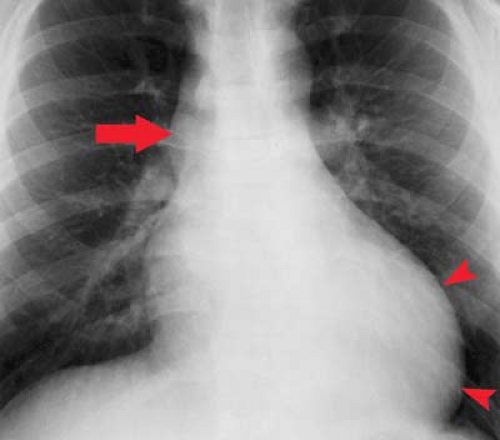
Aorta. Dilatation of the ascending aorta as a result of poststenotic dilatation is seen in approximately 80% of patients with aortic stenosis (Fig. 21.11). It can also be seen in patients older than 50 years when there is tortuosity of the entire aorta or systemic hypertension. Ascending aortic aneurysm (calcific with syphilis, not calcified with Marfan syndrome) is another possibility (Fig. 21.13). A ductus bump adjacent to the aortic knob can be an indication of patent ductus arteriosus.
Azygos vein dilatation (>6 mm on upright PA or >1 cm on supine radiograph) is seen with intravascular volume expansion, elevated central venous pressure, and right heart failure (Fig. 21.21; see Fig. 22.16). Additional causes include the Valsalva maneuver, pregnancy, renal failure, vena cava obstruction, or azygos continuation of the IVC. Dilatation of the SVC often accompanies volume expansion or elevated central venous pressure but is more difficult to detect with certainty.
Cardiac Calcifications
Coronary Calcification. Radiographs commonly demonstrate coronary artery calcification in a 3-cm triangle along the upper left heart border, called the “CAC” (coronary artery calcification) triangle (see Fig. 22.1). If chest pain and coronary calcification are present, there is a 94% chance the patient will have occlusive coronary artery disease at angiography. Fluoroscopic detection of coronary calcification actually has higher sensitivity and specificity in screening asymptomatic individuals than does exercise tolerance testing. In symptomatic patients, the detection of coronary calcification approaches exercise tolerance testing in sensitivity and exceeds exercise tolerance testing in specificity. More than 82% of the patients with fluoroscopically demonstrated coronary artery calcification and positive exercise tolerance testing have significant coronary artery disease at angiography. Calcifications have more significance when seen in patients younger than 60 years of age. Heavier and more extensive calcification correlates with more severe coronary disease. Detection of coronary calcification helps to differentiate patients with ischemic, from those with nonischemic, cardiomyopathy.
Valvular calcification is seen in 85% of patients with acquired valvular disease but is rarely detected in patients younger than 20 years of age. Aortic valve calcification is highly suggestive of valve disease. Calcific aortic stenosis is most often degenerative or atherosclerotic in origin and is usually seen in older males. Extensive aortic annulus calcification is atherosclerotic in nature and has been associated with conduction blocks.
Mitral valve calcification is highly suggestive of rheumatic valvular disease and is seen on chest radiograph in approximately 40% of patients with mitral stenosis. It is even more common in patients with stenosis and regurgitation. Atherosclerotic calcification of the mitral annulus occurs in approximately 10% of the elderly population (Fig. 21.14). It appears
as circular, ovoid, or C- or J-shaped calcification in the mitral annulus and can lead to mitral valve incompetence.
as circular, ovoid, or C- or J-shaped calcification in the mitral annulus and can lead to mitral valve incompetence.
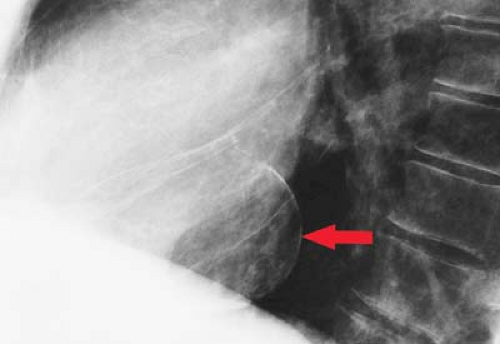 Figure 21.15. Calcified Ventricular Pseudoaneurysm on Lateral CXR. Thin, curvilinear calcification along the posterior wall of the LV (arrow) is indicative of a ventricular pseudoaneurysm. |
Sinus of Valsalva aneurysm calcification is seen as a curvilinear density anterior and lateral to the ascending aorta.
Calcified ligamentum arteriosum is seen as a linear calcification in the aortopulmonary window connecting the top of the left PA to the floor of the aortic arch.
Calcified LA. Thin curvilinear calcification in the wall of the LA is usually associated with mitral stenosis, left atrial enlargement, atrial fibrillation, and left atrial thrombus.
Calcified pericardium is typically anterior and inferior in location. It can be single or double layered and is associated with a high incidence of constrictive pericardial hemodynamics. Causes include viral, hemorrhagic, and tuberculous pericarditis as well as postsurgical scarring.
Calcified Infarct. Dystrophic calcification may occur in the myocardial wall from prior myocardial infarction.
Calcified Ventricular Aneurysm. Thin curvilinear calcification anterolaterally near the apex is most often seen with true aneurysms (see Figs. 22.10, 22.40). Posterior curvilinear calcification is usually seen in pseudoaneurysms (Fig. 21.15).
Calcified thrombus is seen as clumpy calcification in the LA or, less commonly, in the LV.
Calcified PAs. Thin eggshell-like calcification in the walls of the PAs is virtually diagnostic of long-standing pulmonary arterial hypertension (see Figs. 22.22, 22.23).
Tumors. Rounded or stippled calcifications are seen occasionally in atrial myxomas and rarely in other cardiac neoplasms (see Figs. 22.41 to 22.44).
Pulmonary Vascularity
The lungs have dual blood supply with PAs and systemic bronchial arteries.
Pulmonary Arteries. Increased circulation from left-to-right shunts results in enlargement of the main and hilar PAs with increased blood flow to the upper and lower lobes. Asymmetrical blood flow can be seen with pulmonary hypoplasia, Swyer–James syndrome, and congenital lesions such as pulmonary stenosis (increased to the left lung) or tetralogy of Fallot, which is increased to the right lung (Fig. 21.16).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree