■
Introduction
More than 8 million Americans each year present to the emergency department (ED) with acute chest pain. This poses a diagnostic challenge to physicians, who must distinguish patients with life-threatening conditions, including acute coronary syndrome (ACS), aortic dissection, and pulmonary embolism, from those who have chest pain related to more benign causes.
Most patients with chest pain are discharged with a diagnosis of noncardiac origin. Only 12% to 15% of patients with chest pain have ACS, which includes diagnoses such as unstable angina, non-ST elevation myocardial infarction (NSTEMI), and ST elevation myocardial infarction (STEMI).
Routine workup for chest pain is estimated to cost approximately $8 billion each year and often requires admission of patients who ultimately have a non–life-threatening or noncardiac cause of chest pain. Despite advances in diagnostic tools, about 2% to 4% of patients are misdiagnosed and discharged home with ACS, and these patients have twice the mortality rate as those who are admitted and treated with the standard of care. These deficiencies highlight the need for improved algorithms to evaluate patients with acute chest pain. Noninvasive imaging has the potential to rule out ACS in select patients to prevent admission and additional cardiac testing.
■
Standard Approach in Diagnosing Acute Coronary Syndrome
ACS is a heterogeneous syndrome ranging from unstable angina to myocardial infarction (MI; NSTEMI or STEMI), which is usually caused by a ruptured atherosclerotic plaque and thrombus formation that significantly narrows or occludes a coronary artery. Symptoms arise when the oxygen demand of myocardium exceeds supply delivered by coronary vessels, causing reversible myocardial ischemia (unstable angina) or infarction (NSTEMI or STEMI).
The most common clinical presentation is chest pain, which is relatively nonspecific for ACS. Chest pain can also represent other life-threatening conditions, such as aortic dissection, pulmonary embolism, tension pneumothorax, cardiac tamponade, and mediastinitis, or it can represent more common non–life-threatening conditions such as gastroesophageal reflux disease, musculoskeletal pain, and pneumonia.
On admission, the initial history and physical examination are helpful in identifying factors to increase suspicion of or exclude ACS as the cause of chest pain. Chest pain from myocardial ischemia or MI is often gradual in onset and is described as chest discomfort, and radiation to the left arm or lower jaw is more specific to ACS. Other nonspecific symptoms include diaphoresis, nausea and vomiting, dyspnea, syncope, and palpitations. Caution should be taken with patients who are older, female, or diabetic because they may present with atypical symptoms such as weakness, dyspnea, and abdominal pain, and consequently this population is at higher risk for missed diagnosis and delay in care. Pain that responds to nitroglycerin is often thought to have a cardiac cause (or esophageal spasm), but this is not a reliable indicator to distinguish cardiac pain from noncardiac chest pain. Although the presence of cardiac risk factors or known coronary artery disease (CAD) increases the risk for ACS, it is a poor predictor in an acute setting because chest pain itself has a much higher predictive value for ACS than the presence of cardiac risk factors.
All patients undergo 12-lead electrocardiography to assess for evidence of ischemia or infarction, such as new ST elevation (the most specific finding for ACS) or depression in two anatomically contiguous leads, Q waves >30 ms, and inverted T waves in precordial leads. Ischemic changes may be masked in patients who have baseline electrocardiographic abnormalities from hypertrophy, bundle branch block, or ventricular pacing. In most patients with ACS, the electrocardiogram (ECG) is normal or nondiagnostic, so it is recommended that the patient be placed on telemetry with serial ECGs obtained every 5 to 10 minutes to assess for ongoing ischemia in suspected cases of ACS.
Serum biomarkers are also drawn to assess for myocardial injury. Many serum biomarkers have been studied in the setting of ACS (e.g., troponin, creatine kinase-muscle/brain [CK-MB], natriuretic peptides, C-reactive protein [CRP], myeloperoxidase), but cardiac troponin is the most commonly used biomarker in EDs because it is widely available and is highly sensitive and specific for myocardial injury. However, myocardial injury is not specific to ACS. Troponin levels can be elevated in the setting of trauma, renal failure, sepsis, hypertensive emergency, pulmonary embolism, and other conditions. In the setting of ACS, troponin level elevation may not be detected until 4 to 6 hours after the onset of symptoms, so current guidelines recommend that serial troponin levels be checked every 6 to 8 hours to improve detection of ACS. High-sensitivity troponin assays are being developed to detect infarction earlier in ACS, but this will increase the number of false-positives and potentially lead to unnecessary additional testing.
After obtaining the history, physical, initial ECG, and cardiac biomarkers, the need for further studies—noninvasive or invasive imaging—is determined by risk stratification.
■
Patient Stratification in the Evaluation of Acute Coronary Syndrome
From a noninvasive imaging perspective, patients can be divided into three groups based on their risk for ACS and need for further evaluation and workup. The first group of patients consists of those who have high-risk or definite ACS based on the physical examination, ECG, and cardiac biomarkers. These patients are admitted and referred for invasive coronary angiography and possible percutaneous or surgical intervention. Noninvasive imaging is unnecessary in these patients because it will not change the need for catheterization.
The second group of patients consists of those who have a clear noncardiac and non–life-threatening diagnosis, with a normal ECG and cardiac biomarkers, in whom ACS can be confidently excluded. These patients can safely be discharged from the ED without the need for observation or cardiac imaging.
The third group of patients is the largest and consists of those who fall in between the two other groups. These patients often have nonspecific or atypical chest pain, normal or nondiagnostic ECG, and normal cardiac biomarkers. This group usually represents the patient population at a low to intermediate risk for ACS, and they require further evaluation to include or reasonably exclude ACS. Patients with unstable angina usually fall into this group because, by definition, they have normal cardiac biomarkers and frequently have normal or nondiagnostic ECGs. Patients in this group are usually admitted to an observation unit for serial ECGs and cardiac biomarkers; additional tests with stress testing or noninvasive imaging techniques are performed, depending on patient characteristics.
Multiple scoring systems have been developed to assess clinical risk after an ACS; they are primarily based on the physical examination, history, electrocardiographic findings, and biomarkers. Two widely used scoring systems include the TIMI (Thrombolysis In Myocardial Infarction), which assesses the risk of 14-day mortality and MI after ACS, and GRACE (Global Registry of Acute Coronary Events), which assesses index hospitalization mortality and 6-month mortality after ACS. Although these risk-scoring systems focus on outcomes and are not designed to diagnose ACS, they are often used as part of risk stratification to guide management in individuals with suspected ACS. Patients who are at a higher risk of mortality will often be immediately referred for invasive coronary angiography.
■
Role of Noninvasive Imaging in the Conventional Assessment of Acute Coronary Syndrome
Chest Radiography
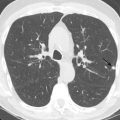
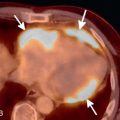
Most patients with ACS have a nondiagnostic chest radiograph. However, a chest radiograph is obtained in virtually all patients who present to the ED with chest pain because it gives a global survey of chest anatomy and can quickly rule out potentially life-threatening pathologies, such as pneumothorax, which require urgent intervention, and more common nonemergent or non–life-threatening pathologies, such as pneumonia or rib fractures. Other abnormalities may give clues to the underlying diagnosis—a widened mediastinum is observed in about 90% of patients with aortic dissection, a wedge-shaped pulmonary infarct (Hampton’s hump) may be present with pulmonary embolism, and pneumomediastinum may be evident with esophageal perforation. Myocardial calcification suggests prior MI ( Fig. 40.1 ), and coronary artery calcification indicates CAD, but these findings are neither sensitive nor specific to ACS.

Exercise Tolerance Test
For many years, the exercise tolerance test (ETT) has been the preferred method in appropriate patients to detect inducible ischemia due to its low cost and wide availability. A standard ETT can be performed on a treadmill or stationary bike, and the ECG is continually monitored to detect ischemic changes as the patient progresses from a resting to a stress state. Although meta-analyses have shown only a modest sensitivity and specificity for detecting CAD, ETT has a negative predictive value greater than 98% for ACS, making it an excellent screening test to rule out ACS. The major limitation of ETT is that a significant number of patients are not suitable candidates because of physical limitations, inability to achieve the necessary heart rate (85% of maximum heart rate), and resting electrocardiographic abnormalities that make ischemic changes difficulty to identify (e.g., ST segment changes, left bundle branch block, arrhythmias). Patients with negative ETT results are usually cleared for discharge, but abnormal or nondiagnostic results require additional cardiac imaging for further evaluation.
Echocardiography
Resting echocardiography can detect ischemia and infarction by visualizing regional wall motion abnormalities, but its usefulness is limited by timing of the examination and size of the ischemic or infarcted myocardium. ACS is a dynamic process and, in the absence of symptoms representing ongoing ischemia or infarction, echocardiography is less likely to visualize wall motion abnormalities. Furthermore, more than 20% of the myocardial thickness must be involved for wall motion abnormalities to appear. Given these limitations, it is not surprising that substantial variability in negative predictive values has been reported, ranging from 57% to 98%. When performed after symptom resolution, echocardiography is a less reliable predictor of future adverse cardiac events and the need for revascularization. Therefore, in the setting of acute chest pain, resting echocardiography is most useful in detecting ACS when the patient is symptomatic or within a few hours of clinical presentation.
Stress echocardiography allows detection of inducible ischemia and can be performed during exercise or, more commonly, using pharmacologic agents (e.g., dobutamine). Multiple studies have found consistently high negative predictive values, ranging from 96% to 100%, which provides support for discharging a patient from the ED with a negative result. Multiple studies have also shown that stress echocardiography gives important prognostic information regarding early and late cardiac events.
Possible advantages of echocardiography include the ability to detect other pathologies such as proximal aortic dissection, large pulmonary embolism, pericarditis, and cardiomyopathy, as well as complications of MI. Echocardiography also provides important cardiac anatomic and physiologic information, including ventricular function, valvular function, and ejection fraction. The use of newer microbubble contrast agents has been shown to improve the ability to detect wall motion abnormalities, which may enhance the sensitivity to detect ACS. Disadvantages include limited availability of sonographers and cardiologists for interpretation during off-hours.
■
Radionuclide Myocardial Perfusion Imaging
Myocardial perfusion imaging (MPI) assesses the distribution of coronary blood flow, which gives direct evidence of ischemia or infarction and clearly identifies the location and extent of abnormality. Old infarctions typically have the same appearance as new infarctions, but they can be distinguished by the presence or absence of cardiac biomarkers.
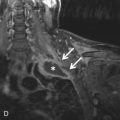
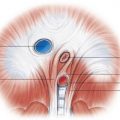
To perform MPI, a cardiac specific tracer material is injected, and imaging is acquired using single-positron emission computed tomography (SPECT), which is demonstrated in Fig. 40.2 . Technetium tracers have mostly replaced the use of thallium tracers because thallium rapidly redistributes and requires imaging within minutes of injection, whereas technetium allows for imaging up to 2 to 4 hours after injection. The timing of the injection is critical because images that are subsequently acquired reflect blood flow and myocardial perfusion at the time of injection. There is evidence that perfusion abnormalities can persist many hours after resolution of symptoms, but MPI has the highest sensitivity when the patient is symptomatic. Current guidelines recommend that the injection should occur while the patient is symptomatic or within 2 hours of symptom resolution.

Rest MPI has been shown to have a sensitivity of up to 97% for obstructive CAD causing ischemia in patients with ACS and has been recommended with class 1 indications for patients with acute chest pain, a nondiagnostic ECG, and normal cardiac biomarkers. Its negative predictive value is 97% to 100%, which makes ACS extremely unlikely in a patient with a normal rest MPI. Randomized clinical trials (RCTs) have found that using rest MPI in diagnostic evaluation results in fewer inappropriate admissions for suspected ACS, a shorter hospital stay, and decreased total costs when compared to standard care.
The use of stress MPI with exercise or pharmacologic agents may increase the sensitivity of detecting ACS compared to rest MPI in stable patients whose symptoms have resolved, although studies have yielded mixed results regarding the superiority of stress MPI compared to rest MPI. Alternatively, vasodilators (e.g., dipyridamole, adenosine) can be administered that preferentially dilate the normal coronary arteries and produce relative perfusion deficits in myocardium supplied by diseased vessels.
Disadvantages of MPI include exposure to radiation when performing the SPECT scan. From a technical standpoint, diagnostic value may be limited by soft tissue densities, which can cause attenuation artifacts that mimic or confound the appearance of perfusion defects. Another weakness is that most institutions have a limited availability of nuclear medicine staff to perform and interpret the study after hours.
Magnetic Resonance Imaging
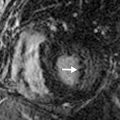
Cardiac MRI has a limited role in the evaluation of acute chest pain. The advantage of MRI is its ability to assess cardiac function, perfusion, and tissue characteristics in a single examination. Unlike other imaging modalities, it can identify areas of reversible and irreversible injury and distinguish acute ( Fig. 40.3 ) from chronic infarction. However, cardiac MRI is not recommended in the setting of acute chest pain due to lack of data, the need to remove the patient from the monitored environment of the emergency room, and the longer duration of the study. The few existing studies show encouraging results. In a study by Kwong et al., 161 patients with suspected ACS underwent MRI to assess perfusion, left ventricular function, and gadolinium enhancement. Sensitivity was 84% and specificity was 85% for detecting ACS. In another study, by Cury et al., the addition of T2-weighted imaging increased the accuracy of detection of ACS from 84% to 93% and improved specificity from 84% to 96%. Larger trials are needed to understand fully the utility of cardiac MRI in the setting of acute chest pain.