Chapter ELEVEN
Cardiac Imaging
LAURA E. HEYNEMAN  CHAPTER EDITOR
CHAPTER EDITOR
LAURA E.
HEYNEMAN
HISTORY
An 18-year-old woman with exertional dyspnea and chest pain.
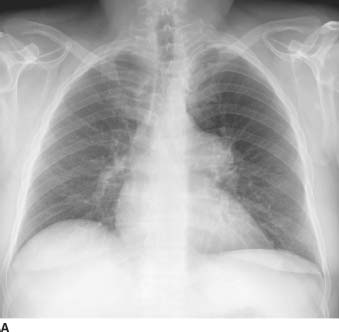
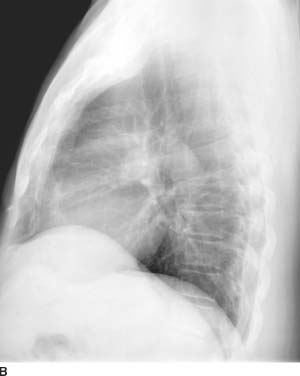
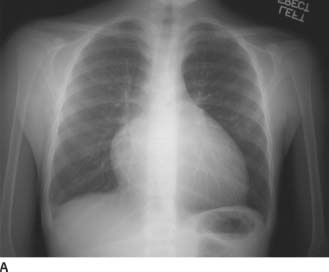
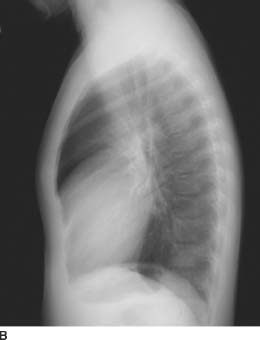
 FIGURES 11-1A and 11-1B Frontal and lateral chest radiographs. There is enlargement of the main pulmonary artery and left pulmonary artery. On the lateral radiograph, there is mild right ventricular prominence and enlargement of the left pulmonary artery.
FIGURES 11-1A and 11-1B Frontal and lateral chest radiographs. There is enlargement of the main pulmonary artery and left pulmonary artery. On the lateral radiograph, there is mild right ventricular prominence and enlargement of the left pulmonary artery.
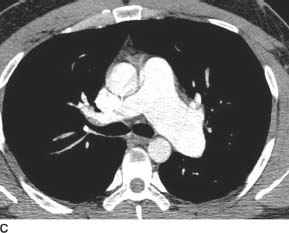
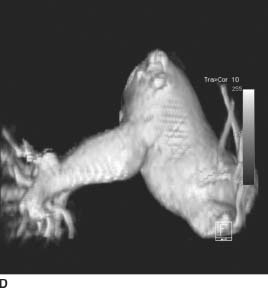
 FIGURES 11-1C and 11-1D Axial (C) and volume-rendered (D) images from contrast-enhanced chest CT. There is enlargement of the main and left pulmonary artery, with a smaller right pulmonary artery. (See color insert)
FIGURES 11-1C and 11-1D Axial (C) and volume-rendered (D) images from contrast-enhanced chest CT. There is enlargement of the main and left pulmonary artery, with a smaller right pulmonary artery. (See color insert)
 Valvular pulmonary stenosis: Enlargement of the main pulmonary artery (PA) and left hilum (with resultant asymmetric pulmonary blood flow to the left) and mild enlargement (with hypertrophy) of the right ventricle are the hallmarks of this entity and make it the most likely diagnosis.
Valvular pulmonary stenosis: Enlargement of the main pulmonary artery (PA) and left hilum (with resultant asymmetric pulmonary blood flow to the left) and mild enlargement (with hypertrophy) of the right ventricle are the hallmarks of this entity and make it the most likely diagnosis.
 Pulmonary artery hypertension (PAH): This entity might be a consideration, because the main and left pulmonary arteries are enlarged. However, the blood flow pattern in PAH would be one of symmetric hilar enlargement with pruning of peripheral vessels. The asymmetric blood flow seen in the case shown above is not characteristic of PAH.
Pulmonary artery hypertension (PAH): This entity might be a consideration, because the main and left pulmonary arteries are enlarged. However, the blood flow pattern in PAH would be one of symmetric hilar enlargement with pruning of peripheral vessels. The asymmetric blood flow seen in the case shown above is not characteristic of PAH.
 Atrial septal defect (ASD): Right ventricular enlargement and increased pulmonary blood flow is a feature of an ASD. In an ASD, all of the pulmonary vessels should be enlarged rather than the asymmetric enlargement as in this case.
Atrial septal defect (ASD): Right ventricular enlargement and increased pulmonary blood flow is a feature of an ASD. In an ASD, all of the pulmonary vessels should be enlarged rather than the asymmetric enlargement as in this case.
 Right pulmonary artery hypoplasia: This entity might be considered because of the discrepancy in size of the two pulmonary arteries. However, in the setting of PA hypoplasia, the right lung is almost always hypoplastic and there is resultant mediastinal shift to the right. In the case provided, the right lung is normal in size and there is no mediastinal shift; therefore, PA hypoplasia is unlikely.
Right pulmonary artery hypoplasia: This entity might be considered because of the discrepancy in size of the two pulmonary arteries. However, in the setting of PA hypoplasia, the right lung is almost always hypoplastic and there is resultant mediastinal shift to the right. In the case provided, the right lung is normal in size and there is no mediastinal shift; therefore, PA hypoplasia is unlikely.
 Pulmonary embolism: This entity can produce asymmetric pulmonary blood flow if there is a large central blood clot. However, no emboli are evident on the CT.
Pulmonary embolism: This entity can produce asymmetric pulmonary blood flow if there is a large central blood clot. However, no emboli are evident on the CT.
DIAGNOSIS
Valvular pulmonary stenosis
KEY FACTS
Clinical
 Valvular pulmonary stenosis is a relatively common congenital heart disorder. Dypsnea and fatigue are the most common symptoms.
Valvular pulmonary stenosis is a relatively common congenital heart disorder. Dypsnea and fatigue are the most common symptoms.
 A loud ejection systolic murmur heard at the upper left sternal border is typical. Electrocardiogram (ECG) findings indicate right ventricular hypertrophy and right axis deviation. Cardiac MR and catheterization show valvular pulmonary stenosis with a systolic pressure gradient across the pulmonic valve.
A loud ejection systolic murmur heard at the upper left sternal border is typical. Electrocardiogram (ECG) findings indicate right ventricular hypertrophy and right axis deviation. Cardiac MR and catheterization show valvular pulmonary stenosis with a systolic pressure gradient across the pulmonic valve.
Radiologic
 The characteristic radiographic findings of valvular pulmonary stenosis can be explained on the basis of the abnormal hemodynamics.
The characteristic radiographic findings of valvular pulmonary stenosis can be explained on the basis of the abnormal hemodynamics.
 During systole, the high pressure within the right ventricle is converted into high-velocity flow, creating a forceful jet in the direction of the main and left pulmonary arteries. Dilatation of the main and left pulmonary artery results.
During systole, the high pressure within the right ventricle is converted into high-velocity flow, creating a forceful jet in the direction of the main and left pulmonary arteries. Dilatation of the main and left pulmonary artery results.
 A low-pressure zone can be created immediately lateral to the central axis of the jet stream. Such reduced pressure has a suction effect, facilitating the leftward flow at the expense of right pulmonary flow. The resultant abnormal flow pattern can be shown by chest radiographs, CTs, pulmonary angiograms, or perfusion lung scan.
A low-pressure zone can be created immediately lateral to the central axis of the jet stream. Such reduced pressure has a suction effect, facilitating the leftward flow at the expense of right pulmonary flow. The resultant abnormal flow pattern can be shown by chest radiographs, CTs, pulmonary angiograms, or perfusion lung scan.
 The high pressure felt by the right ventricle proximal to the stenosis results in right ventricular hypertrophy. On the lateral chest radiograph, the effects on the right ventricle are evident by mild enlargement of the right ventricular shadow. On cross-sectional imaging, enlargement of the right ventricular chamber and thickening of the right ventricular wall are evident.
The high pressure felt by the right ventricle proximal to the stenosis results in right ventricular hypertrophy. On the lateral chest radiograph, the effects on the right ventricle are evident by mild enlargement of the right ventricular shadow. On cross-sectional imaging, enlargement of the right ventricular chamber and thickening of the right ventricular wall are evident.
 Cardiac MR may not only demonstrate the effects on the right ventricle but also the turbulent flow across the pulmonic valve, and phase contrast cardiac MR imaging can calculate the pressure gradient across the valve.
Cardiac MR may not only demonstrate the effects on the right ventricle but also the turbulent flow across the pulmonic valve, and phase contrast cardiac MR imaging can calculate the pressure gradient across the valve.
 The above-mentioned left lateralized pulmonary blood flow pattern of valvular pulmonary stenosis is distinctly different from the bilaterally symmetric increase in pulmonary vascularity seen in an ASD or the symmetric central enlargement with peripheral pruning seen in PAH.
The above-mentioned left lateralized pulmonary blood flow pattern of valvular pulmonary stenosis is distinctly different from the bilaterally symmetric increase in pulmonary vascularity seen in an ASD or the symmetric central enlargement with peripheral pruning seen in PAH.
SUGGESTED READING
Castaner E, Gallardo X, Rimola J, et al. Congenital and acquired pulmonary artery anomalies in the adults: radiologic overview. Radiographics 2006;26:349–371.
Chen JJ, Manning MA, Frazier AA, et al. CT angiography of the cardiac valves: normal, diseased, and postoperative appearances. Radiographics 2009;29:1393-1412.
LAURA E.
HEYNEMAN
HISTORY
A 15-year-old boy with a 5-year history of exertional dyspnea, cyanosis, and intermittent chest pain.
 FIGURES 11-2A and 11-2B Frontal (A) and lateral (B) chest radiographs. There is decreased pulmonary blood flow as evidenced by a small main pulmonary artery, small hila, and small peripheral vessels. Marked right-sided cardiomegaly is seen without evidence of left-sided involvement.
FIGURES 11-2A and 11-2B Frontal (A) and lateral (B) chest radiographs. There is decreased pulmonary blood flow as evidenced by a small main pulmonary artery, small hila, and small peripheral vessels. Marked right-sided cardiomegaly is seen without evidence of left-sided involvement.
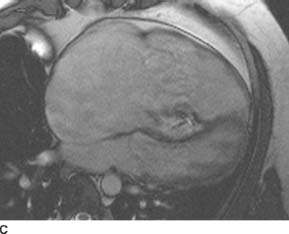
 FIGURE 11-2C Long axis SSFP MR image confirms massive right heart enlargement. The septal leaflet of the tricuspid valve is apically displaced and plastered to the septal wall, and the anterior leaflet of the tricuspid valve is tethered to the anterior wall of the right ventricle. The interventricular septum is bowed toward the left; on cine imaging (not shown), this bowing is more prominent in diastole, indicative of right ventricular volume overload. There is a regurgitant jet of systolic dephasing deep within the right ventricular cavity, indicating the abnormally positioned and regurgitant tricuspid valve. On cine views (not shown), there is virtually no contraction of the base and midportion of the RV, with only a small portion of the RV apex contracting normally. Additionally, there is an ASD.
FIGURE 11-2C Long axis SSFP MR image confirms massive right heart enlargement. The septal leaflet of the tricuspid valve is apically displaced and plastered to the septal wall, and the anterior leaflet of the tricuspid valve is tethered to the anterior wall of the right ventricle. The interventricular septum is bowed toward the left; on cine imaging (not shown), this bowing is more prominent in diastole, indicative of right ventricular volume overload. There is a regurgitant jet of systolic dephasing deep within the right ventricular cavity, indicating the abnormally positioned and regurgitant tricuspid valve. On cine views (not shown), there is virtually no contraction of the base and midportion of the RV, with only a small portion of the RV apex contracting normally. Additionally, there is an ASD.
 Ebstein’s anomaly: This is the best diagnosis based on the decreased pulmonary blood flow, markedly enlarged right heart, and abnormality of the tricuspid valve as demonstrated on the MR.
Ebstein’s anomaly: This is the best diagnosis based on the decreased pulmonary blood flow, markedly enlarged right heart, and abnormality of the tricuspid valve as demonstrated on the MR.
 Pulmonary atresia (without a ventricular septal defect [VSD] and with an incompetent tricuspid valve): The markedly enlarged right heart raises this entity as a possible diagnosis. However, without benefit of surgery, patients with this entity usually die in infancy.
Pulmonary atresia (without a ventricular septal defect [VSD] and with an incompetent tricuspid valve): The markedly enlarged right heart raises this entity as a possible diagnosis. However, without benefit of surgery, patients with this entity usually die in infancy.
 Tetralogy of Fallot (TOF): Despite the similarity of pulmonary oligemia and clinical cyanosis in both entities, the heart in patients with TOF would not attain the size shown above for two reasons. First, the right ventricle in TOF is subjected to increased pressure in the setting of infundibular stenosis; therefore, the right ventricular wall hypertrophies but the cavity does not dilate excessively. Additionally, in patients with TOF, there is a large VSD, which serves as a pathway for blood flow when the pulmonary outflow tract is obstructed. This prevents the right ventricle from markedly dilating. Furthermore, there is no reason for the right atrium to be dilated in TOF. Additionally, in TOF, the tricuspid valve would be normal.
Tetralogy of Fallot (TOF): Despite the similarity of pulmonary oligemia and clinical cyanosis in both entities, the heart in patients with TOF would not attain the size shown above for two reasons. First, the right ventricle in TOF is subjected to increased pressure in the setting of infundibular stenosis; therefore, the right ventricular wall hypertrophies but the cavity does not dilate excessively. Additionally, in patients with TOF, there is a large VSD, which serves as a pathway for blood flow when the pulmonary outflow tract is obstructed. This prevents the right ventricle from markedly dilating. Furthermore, there is no reason for the right atrium to be dilated in TOF. Additionally, in TOF, the tricuspid valve would be normal.
 Tricuspid regurgitation (TR): Acquired tricuspid insufficiency is a strong consideration as TR will result in enlargement of the right atrium and right ventricle (RV), as well as decreased blood flow once the RV fails. The clinical history is helpful in distinguishing the two entities. In an infant, a congenital etiology would be favored. In an older child or young adult, acquired TR is usually caused by acute endocarditis (often in the setting of intravenous drug abuse or an infected central venous catheter), whereas Ebstein’s would result in a more chronic history of symptoms.
Tricuspid regurgitation (TR): Acquired tricuspid insufficiency is a strong consideration as TR will result in enlargement of the right atrium and right ventricle (RV), as well as decreased blood flow once the RV fails. The clinical history is helpful in distinguishing the two entities. In an infant, a congenital etiology would be favored. In an older child or young adult, acquired TR is usually caused by acute endocarditis (often in the setting of intravenous drug abuse or an infected central venous catheter), whereas Ebstein’s would result in a more chronic history of symptoms.
 Cardiomyopathy: Marked cardiomegaly on the radiograph can be seen with a dilated cardiomyopathy, but it would be very unusual for the cardiomegaly to be purely right sided. Additionally, the pulmonary blood flow is rarely decreased in the setting of a dilated cardiomy-opathy. Usually, patients with dilated cardiomyopathy demonstrate pulmonary edema from left ventricular (LV) failure rather than diminished blood flow.
Cardiomyopathy: Marked cardiomegaly on the radiograph can be seen with a dilated cardiomyopathy, but it would be very unusual for the cardiomegaly to be purely right sided. Additionally, the pulmonary blood flow is rarely decreased in the setting of a dilated cardiomy-opathy. Usually, patients with dilated cardiomyopathy demonstrate pulmonary edema from left ventricular (LV) failure rather than diminished blood flow.
 Pericardial effusion: This entity can produce marked enlargement of the cardiac silhouette. Large effusions are usually chronic. Normal pulmonary blood flow is maintained in the setting of a pericardial effusion unless there is tamponade; when tamponade impairs the function of the right heart, pulmonary blood flow will be decreased. Additionally, if there is a pericar-dial effusion, the pericardial fluid collection (and not a markedly enlarged right heart) would be seen on the MR images.
Pericardial effusion: This entity can produce marked enlargement of the cardiac silhouette. Large effusions are usually chronic. Normal pulmonary blood flow is maintained in the setting of a pericardial effusion unless there is tamponade; when tamponade impairs the function of the right heart, pulmonary blood flow will be decreased. Additionally, if there is a pericar-dial effusion, the pericardial fluid collection (and not a markedly enlarged right heart) would be seen on the MR images.
DIAGNOSIS
Ebstein’s anomaly
KEY FACTS
Clinical
 Ebstein’s anomaly can present in the newborn period (with cyanosis and marked cardiomegaly) or later in childhood, usually with cyanosis, dyspnea, chest pain, or arrhythmias.
Ebstein’s anomaly can present in the newborn period (with cyanosis and marked cardiomegaly) or later in childhood, usually with cyanosis, dyspnea, chest pain, or arrhythmias.
 The primary abnormality in Ebstein’s anomaly involves the tricuspid valve. The posterior and septal leaflets of the tricuspid valve are delaminated and apically displaced into the right ventricle. The anterior leaflet is often adherent to the anterior wall of the right ventricle. The septal leaflet may be absent. The overall result is severe tricuspid insufficiency.
The primary abnormality in Ebstein’s anomaly involves the tricuspid valve. The posterior and septal leaflets of the tricuspid valve are delaminated and apically displaced into the right ventricle. The anterior leaflet is often adherent to the anterior wall of the right ventricle. The septal leaflet may be absent. The overall result is severe tricuspid insufficiency.
 The portion of the RV into which the tricuspid leaflets are displaced and the anterior leaflet is tethered do not contract normally. This portion of the RV wall is somewhat redundant and boggy, and the affected portion of the RV acts essentially as a collecting chamber. This is referred to as the “atrialized” portion of the RV.
The portion of the RV into which the tricuspid leaflets are displaced and the anterior leaflet is tethered do not contract normally. This portion of the RV wall is somewhat redundant and boggy, and the affected portion of the RV acts essentially as a collecting chamber. This is referred to as the “atrialized” portion of the RV.
 A right-to-left shunt across an ASD or patent foramen ovale is often present.
A right-to-left shunt across an ASD or patent foramen ovale is often present.
 Standard surgical repair involves replacement of the tricuspid valve, plication of the atrialized right ventricle, and closure of an ASD.
Standard surgical repair involves replacement of the tricuspid valve, plication of the atrialized right ventricle, and closure of an ASD.
Radiologic
 The radiographic findings in Ebstein’s anomaly are highly suggestive of the diagnosis, which can often be established definitively by echocardiography or cardiac MR.
The radiographic findings in Ebstein’s anomaly are highly suggestive of the diagnosis, which can often be established definitively by echocardiography or cardiac MR.
 Gross enlargement of the right ventricle and the right atrium is typical for Ebstein’s anomaly because severe tricuspid insufficiency causes considerable right-sided volume overload without a runoff at the ventricular level.
Gross enlargement of the right ventricle and the right atrium is typical for Ebstein’s anomaly because severe tricuspid insufficiency causes considerable right-sided volume overload without a runoff at the ventricular level.
 Pulmonary oligemia is caused by (1) a marked decrease in forward pulmonary blood flow, (2) severe tricuspid insufficiency, and (3) right-to-left shunting across the atrial septum in those patients in whom there is an ASD.
Pulmonary oligemia is caused by (1) a marked decrease in forward pulmonary blood flow, (2) severe tricuspid insufficiency, and (3) right-to-left shunting across the atrial septum in those patients in whom there is an ASD.
 On MRI, the abnormalities of the tricuspid valve are apparent, with gross enlargement of the right heart. The interventricular septum may be convex toward the left during diastole, indicating right ventricular volume overload.
On MRI, the abnormalities of the tricuspid valve are apparent, with gross enlargement of the right heart. The interventricular septum may be convex toward the left during diastole, indicating right ventricular volume overload.
 When evaluating a patient with Ebstein’s anomaly, a search for an associated ASD should be conducted.
When evaluating a patient with Ebstein’s anomaly, a search for an associated ASD should be conducted.
SUGGESTED READING
Ferguson EC, Krishnamurthy R, Oldham SAA. Classic imaging signs of congenital cardiovascular abnormalities. Radiographics 2007;27:1323–1334.
Leschka S, Oechslin E, Husmann L, et al. Pre- and postoperative evaluation of congenital heart disease in children and adults with 64-section CT. Radiographics 2007;27:829–846.
Cook, AL, Hurwitz LM, Valente AM, Herlong JR. Right heart dilatation in adults: congenital causes. Am J Roentgenol 2007;189:592–601.
LAURA E.
HEYNEMAN
HISTORY
A 2-year-old asymptomatic girl with a cardiac murmur since birth.
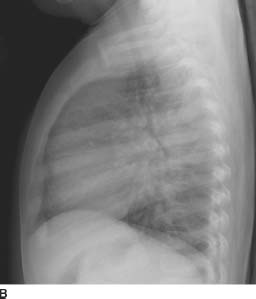
 FIGURES 11-3A and 11-3B Frontal and lateral chest radiographs show increased pulmonary blood flow as evidenced by dilation of all pulmonary vessels including the main pulmonary artery. There is moderate cardiomegaly with predominantly left-sided enlargement. The aortic arch is prominent.
FIGURES 11-3A and 11-3B Frontal and lateral chest radiographs show increased pulmonary blood flow as evidenced by dilation of all pulmonary vessels including the main pulmonary artery. There is moderate cardiomegaly with predominantly left-sided enlargement. The aortic arch is prominent.
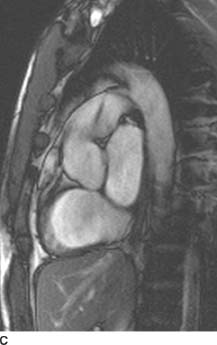
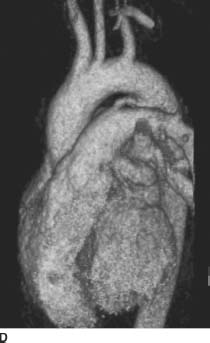
 FIGURES 11-3C and 11-3D Oblique sagittal SSFP MR image (C) performed in a different patient with the same diagnosis demonstrates a flow jet within the main pulmonary artery. This flow jet appears to originate from a connection between the proximal descending aorta and the main pulmonary artery. Volume-rendered image from an MR angiogram (D) in this second patient reveals a connection between the proximal descending aorta and the main pulmonary artery. (See color insert)
FIGURES 11-3C and 11-3D Oblique sagittal SSFP MR image (C) performed in a different patient with the same diagnosis demonstrates a flow jet within the main pulmonary artery. This flow jet appears to originate from a connection between the proximal descending aorta and the main pulmonary artery. Volume-rendered image from an MR angiogram (D) in this second patient reveals a connection between the proximal descending aorta and the main pulmonary artery. (See color insert)
 Patent ductus arteriosus (PDA): This lesion is the correct diagnosis because on the radiograph it results in increased pulmonary blood flow and pure left-sided cardiac enlargement. The transverse aorta may be enlarged. Additionally, the MR images demonstrate the connection between the aorta and the pulmonary artery with the resultant jet of turbulent flow within the pulmonary artery.
Patent ductus arteriosus (PDA): This lesion is the correct diagnosis because on the radiograph it results in increased pulmonary blood flow and pure left-sided cardiac enlargement. The transverse aorta may be enlarged. Additionally, the MR images demonstrate the connection between the aorta and the pulmonary artery with the resultant jet of turbulent flow within the pulmonary artery.
 Ventricular septal defect (VSD): VSDs show increased pulmonary blood flow on a radiograph, but the right ventricle should be enlarged due to the fact that the RV has to accommodate the increased volume of blood coursing across the ventricular septum. Additionally, a VSD would not explain a connection between the aorta and PA as seen on the MR images.
Ventricular septal defect (VSD): VSDs show increased pulmonary blood flow on a radiograph, but the right ventricle should be enlarged due to the fact that the RV has to accommodate the increased volume of blood coursing across the ventricular septum. Additionally, a VSD would not explain a connection between the aorta and PA as seen on the MR images.
 Atrial septal defect (ASD): ASDs demonstrate increased pulmonary blood flow on a radiograph, but enlargement should be purely right-sided. The aortic arch may be small. Furthermore, an ASD would not explain the connection between the aorta and the PA as evidenced by the MR images.
Atrial septal defect (ASD): ASDs demonstrate increased pulmonary blood flow on a radiograph, but enlargement should be purely right-sided. The aortic arch may be small. Furthermore, an ASD would not explain the connection between the aorta and the PA as evidenced by the MR images.
DIAGNOSIS
Patent ductus arteriosus
KEY FACTS
Clinical
 Patients with a large PDA are usually symptomatic in infancy because of congestive heart failure. Older children and adults with a small PDA may be asymptomatic until they develop Eisenmenger’s physiology.
Patients with a large PDA are usually symptomatic in infancy because of congestive heart failure. Older children and adults with a small PDA may be asymptomatic until they develop Eisenmenger’s physiology.
 Because there is a left-to-right shunt with no admixture of oxygenated and deoxygenated blood, PDA patients are not cyanotic.
Because there is a left-to-right shunt with no admixture of oxygenated and deoxygenated blood, PDA patients are not cyanotic.
 A continuous, “machinery-like” murmur is usually heard over the left upper sternal border.
A continuous, “machinery-like” murmur is usually heard over the left upper sternal border.
Radiologic
 There is increased pulmonary blood flow with dilation of all pulmonary vessels due to the substantial left-to-right shunt.
There is increased pulmonary blood flow with dilation of all pulmonary vessels due to the substantial left-to-right shunt.
 In the absence of congestive heart failure or Eisen-menger’s physiology, there is usually pure left-sided cardiomegaly with dilatation of both great arteries. The left atrium and left ventricle dilate to accommodate increased circulating blood volumes. The right heart is spared until the development of Eisenmenger’s physiology, at which point right ventricular hypertrophy may be evident.
In the absence of congestive heart failure or Eisen-menger’s physiology, there is usually pure left-sided cardiomegaly with dilatation of both great arteries. The left atrium and left ventricle dilate to accommodate increased circulating blood volumes. The right heart is spared until the development of Eisenmenger’s physiology, at which point right ventricular hypertrophy may be evident.
 On cross-sectional imaging, a direct communication between the proximal descending thoracic aorta (distal to the origin of the left subclavian artery) and the main pulmonary artery may be visualized. On cine bright blood MR imaging, a continuous jet of turbulent flow may be identified within the main pulmonary artery.
On cross-sectional imaging, a direct communication between the proximal descending thoracic aorta (distal to the origin of the left subclavian artery) and the main pulmonary artery may be visualized. On cine bright blood MR imaging, a continuous jet of turbulent flow may be identified within the main pulmonary artery.
SUGGESTED READING
Frank L, Dillman JR, Parish V, et al. Cardiovascular MR imaging of conotruncal anomalies. Radiographics 2010;30:1069–1094.
Martinez-Jimenez S, Heyneman LE, McAdams HP, et al. Nonsurgi-cal extracardiac vascular shunts in the thorax: clinical and imaging characteristics. Radiographics 2010;30:e41.
LAURA E.
HEYNEMAN
HISTORY
A 2-day-old child with tachypnea, cyanosis, bilateral crackling rales, and a heart murmur.
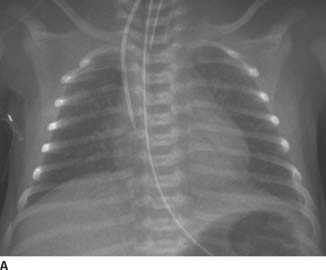
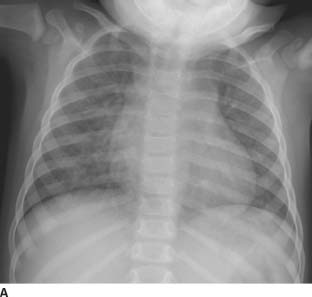
 FIGURE 11-4A Frontal chest radiograph demonstrates an endotracheal tube, an enteric tube, a right internal jugular central venous catheter, diffuse pulmonary edema, and a normal cardiac size.
FIGURE 11-4A Frontal chest radiograph demonstrates an endotracheal tube, an enteric tube, a right internal jugular central venous catheter, diffuse pulmonary edema, and a normal cardiac size.
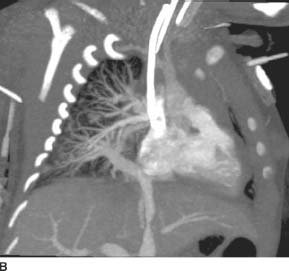
 FIGURE 11-4B Oblique coronal MIP image from the venous phase of a pulmonary arteriogram shows that the pulmonary veins converge into a common collecting vessel; this common collecting vessel traverses the diaphragm and empties into the portal vein. Additional images (not shown) confirm that all of the pulmonary veins from both lungs drain into the common collecting vessel.
FIGURE 11-4B Oblique coronal MIP image from the venous phase of a pulmonary arteriogram shows that the pulmonary veins converge into a common collecting vessel; this common collecting vessel traverses the diaphragm and empties into the portal vein. Additional images (not shown) confirm that all of the pulmonary veins from both lungs drain into the common collecting vessel.
 Total anomalous pulmonary venous return, obstructed variety: In this entity, patients are cyanotic, have pulmonary edema, and have a normal heart size. These features, along with the pulmonary angiogram findings, make this the correct diagnosis.
Total anomalous pulmonary venous return, obstructed variety: In this entity, patients are cyanotic, have pulmonary edema, and have a normal heart size. These features, along with the pulmonary angiogram findings, make this the correct diagnosis.
 Hypoplastic left heart syndrome: This entity is a cause of neonatal cyanotic heart disease and severe pulmonary edema. There are severe left-sided obstructive lesions with a right-to-left shunt at the ductal level and a left-to-right shunt at the atrial level. However, two facts make this diagnosis unlikely in this patient: (1) the cardiac size is usually markedly enlarged, and (2) the pulmonary veins drain normally into the left atrium. Neither of these features is present in the case shown.
Hypoplastic left heart syndrome: This entity is a cause of neonatal cyanotic heart disease and severe pulmonary edema. There are severe left-sided obstructive lesions with a right-to-left shunt at the ductal level and a left-to-right shunt at the atrial level. However, two facts make this diagnosis unlikely in this patient: (1) the cardiac size is usually markedly enlarged, and (2) the pulmonary veins drain normally into the left atrium. Neither of these features is present in the case shown.
 Left-sided obstructive lesions: These lesions—for example, aortic stenosis (AS) and coarctation of the aorta—may present in the neonatal period with severe congestive heart failure. However, two facts make this diagnosis unlikely: (1) patients with these lesions are not cyanotic, and (2) the heart is usually enlarged from left-sided failure, unless the obstruction is very proximal on the left (e.g., cor triatriatum).
Left-sided obstructive lesions: These lesions—for example, aortic stenosis (AS) and coarctation of the aorta—may present in the neonatal period with severe congestive heart failure. However, two facts make this diagnosis unlikely: (1) patients with these lesions are not cyanotic, and (2) the heart is usually enlarged from left-sided failure, unless the obstruction is very proximal on the left (e.g., cor triatriatum).
 Systemic arteriovenous (AV) fistulae: These lesions—for example, vein of Galen malformation or hepatic hemangioendothelioma—can cause frank congestive heart failure early in life. However, this diagnosis is unlikely for two reasons: (1) patients with these lesions are not cyanotic, and (2) the heart is markedly enlarged due to high output failure.
Systemic arteriovenous (AV) fistulae: These lesions—for example, vein of Galen malformation or hepatic hemangioendothelioma—can cause frank congestive heart failure early in life. However, this diagnosis is unlikely for two reasons: (1) patients with these lesions are not cyanotic, and (2) the heart is markedly enlarged due to high output failure.
DIAGNOSIS
Total anomalous pulmonary venous return, obstructed variety
KEY FACTS
Clinical
 In the infradiaphragmatic, obstructed version of total anomalous pulmonary venous return, the common collecting pulmonary vein usually empties into the hepatic portal circulation. As a result, all pulmonary blood flow must course through the relatively high-resistance hepatic sinusoids before reconstituting within the inferior vena cava. Additionally, there is often a focal stenosis of the collecting vein at the level of the diaphragm; this results in further obstruction.
In the infradiaphragmatic, obstructed version of total anomalous pulmonary venous return, the common collecting pulmonary vein usually empties into the hepatic portal circulation. As a result, all pulmonary blood flow must course through the relatively high-resistance hepatic sinusoids before reconstituting within the inferior vena cava. Additionally, there is often a focal stenosis of the collecting vein at the level of the diaphragm; this results in further obstruction.
 Neonates with severe congestive heart failure due to this disorder present with cyanosis, tachypnea, and crackling rales. Frequently, the rales are loud enough to obscure the cardiac murmur. Because the heart size is normal, newborn respiratory distress syndrome can be mistakenly diagnosed.
Neonates with severe congestive heart failure due to this disorder present with cyanosis, tachypnea, and crackling rales. Frequently, the rales are loud enough to obscure the cardiac murmur. Because the heart size is normal, newborn respiratory distress syndrome can be mistakenly diagnosed.
 Helpful clues to the correct diagnosis include (1) normal heart size, (2) severe pulmonary edema, and (3) deepening cyanosis with feeding.
Helpful clues to the correct diagnosis include (1) normal heart size, (2) severe pulmonary edema, and (3) deepening cyanosis with feeding.
 Emergency surgical repair is indicated and consists of anastomosis of the pulmonary venous confluence to the left atrium. Thereafter, the ASD is repaired.
Emergency surgical repair is indicated and consists of anastomosis of the pulmonary venous confluence to the left atrium. Thereafter, the ASD is repaired.
Radiologic
 There is severe pulmonary edema from the obstruction of pulmonary venous outflow.
There is severe pulmonary edema from the obstruction of pulmonary venous outflow.
 All patients with total anomalous pulmonary venous return have a shunt that allows admixture of oxygenated and deoxygenated blood. This shunt is usually in the form of an ASD.
All patients with total anomalous pulmonary venous return have a shunt that allows admixture of oxygenated and deoxygenated blood. This shunt is usually in the form of an ASD.
 In this entity, the heart is normal in size because of the severe obstruction to pulmonary venous outflow; as a result of the obstruction, not enough blood returns to the right heart to cause dilation.
In this entity, the heart is normal in size because of the severe obstruction to pulmonary venous outflow; as a result of the obstruction, not enough blood returns to the right heart to cause dilation.
SUGGESTED READING
Ferguson EC, Krishnamurthy R, Oldham SAA. Classic imaging signs of congenital cardiovascular abnormalities. Radiographics 2007;27:1323–1334.
Leschka S, Oechslin E, Husmann L, et al. Pre- and postoperative evaluation of congenital heart disease in children and adults with 64-section CT. Radiographics 2007;27:829–846.
LAURA E.
HEYNEMAN
HISTORY
A newborn boy noted to be extremely cyanotic, tachypneic, and tachycardic.
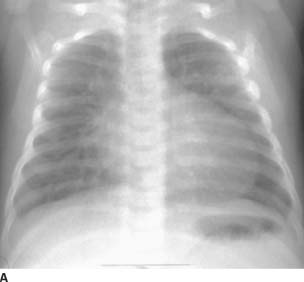
 FIGURE 11-5A Frontal chest radiograph. The pulmonary blood flow is increased. Moderate cardiomegaly with an egg-shaped cardiac contour is seen. The superior mediastinum is narrow.
FIGURE 11-5A Frontal chest radiograph. The pulmonary blood flow is increased. Moderate cardiomegaly with an egg-shaped cardiac contour is seen. The superior mediastinum is narrow.
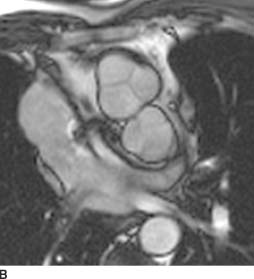
 FIGURE 11-5B Axial SSFP MR image in a different patient with the same disorder. The aortic valve with the sinuses of Valsalva is anterior and to the right of the pulmonic valve.
FIGURE 11-5B Axial SSFP MR image in a different patient with the same disorder. The aortic valve with the sinuses of Valsalva is anterior and to the right of the pulmonic valve.
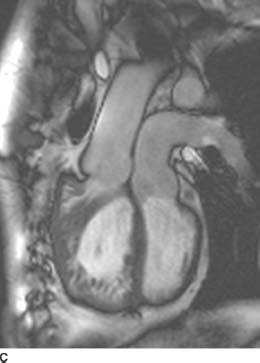
 FIGURE 11-5C Oblique sagittal SSFP MR image shows that the aorta arises off of the anterior, heavily trabeculated right ventricle and the pulmonary artery arises off of the posterior, smooth-walled left ventricle. Note that there is right ventricular hypertrophy as a result of the right ventricle having to pump against systemic pressures.
FIGURE 11-5C Oblique sagittal SSFP MR image shows that the aorta arises off of the anterior, heavily trabeculated right ventricle and the pulmonary artery arises off of the posterior, smooth-walled left ventricle. Note that there is right ventricular hypertrophy as a result of the right ventricle having to pump against systemic pressures.
 D-loop transposition of the great arteries (D-TGA):This diagnosis is the best choice, given the history, the radiographic findings of increased blood flow, and the “egg on a string” appearance of the cardiomediastinal silhouette. The characteristic findings are present on the MR, confirming the diagnosis.
D-loop transposition of the great arteries (D-TGA):This diagnosis is the best choice, given the history, the radiographic findings of increased blood flow, and the “egg on a string” appearance of the cardiomediastinal silhouette. The characteristic findings are present on the MR, confirming the diagnosis.
 Large ventricular septal defect (VSD): Patients with a VSD should not be cyanotic. Additionally, newborns with a VSD usually do not have increased pulmonary blood flow due to physiologic high pulmonary vascular resistance. Shunting in patients with a VSD is most often insignificant until pulmonary resistance falls at 6 weeks of life; it is at that point that their chest radiographs reflect increased pulmonary blood flow from left-to-right shunting. Furthermore, patients with a VSD do not have a narrow mediastinum because their great vessels are normally related.
Large ventricular septal defect (VSD): Patients with a VSD should not be cyanotic. Additionally, newborns with a VSD usually do not have increased pulmonary blood flow due to physiologic high pulmonary vascular resistance. Shunting in patients with a VSD is most often insignificant until pulmonary resistance falls at 6 weeks of life; it is at that point that their chest radiographs reflect increased pulmonary blood flow from left-to-right shunting. Furthermore, patients with a VSD do not have a narrow mediastinum because their great vessels are normally related.
 Hypoplastic left heart syndrome: Patients with this syndrome are also cyanotic neonates, but they are almost always in profound heart failure with pulmonary edema (and do not have increased pulmonary flow, as in the case presented). Furthermore, their hearts tend to be larger than those seen in the cases shown. These factors make this diagnosis unlikely.
Hypoplastic left heart syndrome: Patients with this syndrome are also cyanotic neonates, but they are almost always in profound heart failure with pulmonary edema (and do not have increased pulmonary flow, as in the case presented). Furthermore, their hearts tend to be larger than those seen in the cases shown. These factors make this diagnosis unlikely.
 Total anomalous pulmonary venous return with obstruction: These patients are also cyanotic neonates, but their radiographs usually demonstrate severe pulmonary edema with a normal cardiac size.
Total anomalous pulmonary venous return with obstruction: These patients are also cyanotic neonates, but their radiographs usually demonstrate severe pulmonary edema with a normal cardiac size.
DIAGNOSIS
D-loop transposition of great arteries (D-TGA)
KEY FACTS
Clinical
 D-TGA accounts for 4% of congenital cardiac defects in children.
D-TGA accounts for 4% of congenital cardiac defects in children.
 D-TGA is the most common lesion to present with severe cyanosis immediately after birth.
D-TGA is the most common lesion to present with severe cyanosis immediately after birth.
 The basic pathologic anatomy of D-TGA is as follows: The aorta arises from the right ventricle and the pulmonary artery arises from the left ventricle. This results in two “closed loop” circulations that are incompatible with life without the presence of a shunt that allows admixture of oxygenated and deoxygenated blood. The shunt may be in the form of a patent foramen ovale, an ASD, or a PDA. However, these shunts may only temporize the situation, and emergent balloon septostomy may need to be performed to allow adequate mixing of pulmonary and systemic circulations.
The basic pathologic anatomy of D-TGA is as follows: The aorta arises from the right ventricle and the pulmonary artery arises from the left ventricle. This results in two “closed loop” circulations that are incompatible with life without the presence of a shunt that allows admixture of oxygenated and deoxygenated blood. The shunt may be in the form of a patent foramen ovale, an ASD, or a PDA. However, these shunts may only temporize the situation, and emergent balloon septostomy may need to be performed to allow adequate mixing of pulmonary and systemic circulations.
 Current definitive repair is the Jatene procedure, in which the aorta and pulmonary vessels are switched. Previously, Mustard and Senning atrial baffle procedures were performed. In the Mustard or Senning procedures, the right venticle remained connected to the aorta. As a result, these patients would demonstrate marked right ventricular hypertrophy and eventually would develop right ventricular failure.
Current definitive repair is the Jatene procedure, in which the aorta and pulmonary vessels are switched. Previously, Mustard and Senning atrial baffle procedures were performed. In the Mustard or Senning procedures, the right venticle remained connected to the aorta. As a result, these patients would demonstrate marked right ventricular hypertrophy and eventually would develop right ventricular failure.
Radiologic
 On the radiograph, there is increased pulmonary blood flow with moderate (right greater than left) cardiomegaly.
On the radiograph, there is increased pulmonary blood flow with moderate (right greater than left) cardiomegaly.
 The “egg on the string” appearance of the cardiomediastinal contour is due to several factors. The “egg” is due to cardiomegaly; the right heart is pumping against the high systemic vascular resistance and is enlarged to a greater degree than the left heart. Hence, a globular cardiac contour results. The “string” is due to a narrow mediastinum. The mediastinum is narrowed due to the fact that the aorta and main pulmonary artery have an abnormal relationship and have a more overlapping configuration than normal. Additionally, the mediastinum is narrowed due to involution of the thymus from stress.
The “egg on the string” appearance of the cardiomediastinal contour is due to several factors. The “egg” is due to cardiomegaly; the right heart is pumping against the high systemic vascular resistance and is enlarged to a greater degree than the left heart. Hence, a globular cardiac contour results. The “string” is due to a narrow mediastinum. The mediastinum is narrowed due to the fact that the aorta and main pulmonary artery have an abnormal relationship and have a more overlapping configuration than normal. Additionally, the mediastinum is narrowed due to involution of the thymus from stress.
 On cross-sectional imaging, the diagnosis of D-TGA may be made when the aortic valve is identified anterior and to the right of the pulmonic valve. The aorta is connected to the anterior, trabeculated right ventricle and the pulmonary artery arises from the more posterior, smooth-walled left ventricle.
On cross-sectional imaging, the diagnosis of D-TGA may be made when the aortic valve is identified anterior and to the right of the pulmonic valve. The aorta is connected to the anterior, trabeculated right ventricle and the pulmonary artery arises from the more posterior, smooth-walled left ventricle.
SUGGESTED READING
Ferguson E, Krishnamurthy R, Oldham SAA. Classic imaging signs of congenital cardiovascular abnormalities. Radiographics 2007;27: 1323–1334.
Warnes, CA. Transposition of the great arteries. Circulation 2006;114:2699–2709.
GLEN A.
TOOMAYAN
HISTORY
A 14-year-old male presenting with a syncopal episode requiring cardiopulmonary resuscitation.
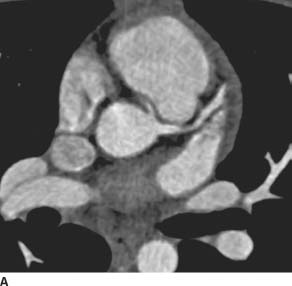
 FIGURE 11-6A Axial image from an EKG-gated contrast-enhanced cardiac CT reveals an anomalous origin of the RCA off of the left coronary sinus. The visualized portion of the RCA courses between the aorta and the right ventricular outflow tract.
FIGURE 11-6A Axial image from an EKG-gated contrast-enhanced cardiac CT reveals an anomalous origin of the RCA off of the left coronary sinus. The visualized portion of the RCA courses between the aorta and the right ventricular outflow tract.
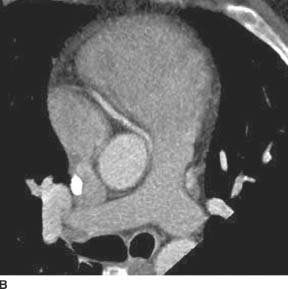
 FIGURE 11-6B Oblique axial CTA image along the axis of the main pulmonary artery demonstrates the anomalous origin of the right coronary artery from the left coronary sinus and its anomalous interarterial course as it heads toward its normal location within the right atrioventricular groove.
FIGURE 11-6B Oblique axial CTA image along the axis of the main pulmonary artery demonstrates the anomalous origin of the right coronary artery from the left coronary sinus and its anomalous interarterial course as it heads toward its normal location within the right atrioventricular groove.
 Anomalous origin of the right coronary artery (RCA) from the left coronary sinus with interarterial course:There is no differential diagnosis. Coronary CT arteriography demonstrates both the right and left coronary arteries to arise from the left coronary sinus. The RCA passes between the ascending aorta and the main pulmonary artery.
Anomalous origin of the right coronary artery (RCA) from the left coronary sinus with interarterial course:There is no differential diagnosis. Coronary CT arteriography demonstrates both the right and left coronary arteries to arise from the left coronary sinus. The RCA passes between the ascending aorta and the main pulmonary artery.
DIAGNOSIS
Anomalous origin of the right coronary artery from the left coronary sinus with interarterial course
KEY FACTS
Clinical
 Coronary artery anomalies have been identified in 0.3% to 1% of healthy individuals and are an important cause of myocardial ischemia, syncope, and sudden death in young adults. This particular anomaly is very rare, occurring in 0.019% to 0.17% of adults.
Coronary artery anomalies have been identified in 0.3% to 1% of healthy individuals and are an important cause of myocardial ischemia, syncope, and sudden death in young adults. This particular anomaly is very rare, occurring in 0.019% to 0.17% of adults.
 Coronary artery anomalies may be classified as anomalies of coronary artery origin, course, or termination.
Coronary artery anomalies may be classified as anomalies of coronary artery origin, course, or termination.
 They may also be classified as hemodynamically significant or insignificant.
They may also be classified as hemodynamically significant or insignificant.
 Hemodynamically significant anomalies result in abnormal myocardial perfusion and are associated with an increased risk of myocardial ischemia and sudden death.
Hemodynamically significant anomalies result in abnormal myocardial perfusion and are associated with an increased risk of myocardial ischemia and sudden death.
 Hemodynamically significant anomalies include anomalous origin of a coronary artery from the pulmonary artery, anomalies of coronary artery origin with an interarterial course (between the aorta and pulmonary artery), and occasionally myocardial bridges.
Hemodynamically significant anomalies include anomalous origin of a coronary artery from the pulmonary artery, anomalies of coronary artery origin with an interarterial course (between the aorta and pulmonary artery), and occasionally myocardial bridges.
 Treatment options for an anomalous coronary artery with an interarterial course include coronary artery bypass grafting or unroofing or reimplantation of the coronary artery.
Treatment options for an anomalous coronary artery with an interarterial course include coronary artery bypass grafting or unroofing or reimplantation of the coronary artery.
Radiologic
 Coronary artery anomalies were traditionally diagnosed with conventional angiography. However, only 53% of coronary anomalies detected by CT angiogram (CTA) are correctly identified with conventional angiography. The RCA normally arises from the anterior right coronary sinus and passes posterior and to the right of the main pulmonary artery before descending within the right atrioventricular groove.
Coronary artery anomalies were traditionally diagnosed with conventional angiography. However, only 53% of coronary anomalies detected by CT angiogram (CTA) are correctly identified with conventional angiography. The RCA normally arises from the anterior right coronary sinus and passes posterior and to the right of the main pulmonary artery before descending within the right atrioventricular groove.
 The left coronary artery (LCA) normally arises from the posterior left coronary sinus and passes posterior and to the left of the main pulmonary artery. The LCA normally bifurcates into the left circumflex (LCx) and left anterior descending (LAD) coronary arteries.
The left coronary artery (LCA) normally arises from the posterior left coronary sinus and passes posterior and to the left of the main pulmonary artery. The LCA normally bifurcates into the left circumflex (LCx) and left anterior descending (LAD) coronary arteries.
 The LAD normally passes to the left of the main pulmonary artery before descending within the anterior interventricular groove. The LCx normally descends in the left atrioventricular groove.
The LAD normally passes to the left of the main pulmonary artery before descending within the anterior interventricular groove. The LCx normally descends in the left atrioventricular groove.
 Anomalies of coronary artery origin include (1) RCA arising from the left coronary sinus, (2) LCA, LCx, or LAD arising from the right coronary sinus, (3) either a coronary artery or a coronary artery branch arising from the noncoronary sinus, or (4) a coronary artery (usually the left) arising from the main pulmonary artery.
Anomalies of coronary artery origin include (1) RCA arising from the left coronary sinus, (2) LCA, LCx, or LAD arising from the right coronary sinus, (3) either a coronary artery or a coronary artery branch arising from the noncoronary sinus, or (4) a coronary artery (usually the left) arising from the main pulmonary artery.
 The anomalous artery may then take one of four courses—(1) interarterial (between the aorta and pulmonary artery), (2) retroaortic, (3) prepulmonic, or (4) subpulmonic. The interarterial course is associated with myocardial ischemia, syncope, and sudden cardiac death and requires surgical treatment.
The anomalous artery may then take one of four courses—(1) interarterial (between the aorta and pulmonary artery), (2) retroaortic, (3) prepulmonic, or (4) subpulmonic. The interarterial course is associated with myocardial ischemia, syncope, and sudden cardiac death and requires surgical treatment.
 Potential mechanisms for sudden death with an interarterial course of an anomalous coronary artery include cyclical compression between the aorta and pulmonary artery, stretching of the intramural segment, or kinking of its origin due to an abnormal acute angulation of the takeoff from the aorta. Furthermore, when the coronary artery arises anomalously off of the aorta, the orifice is often slit-like and more easily compressed by the muscular wall of the aorta.
Potential mechanisms for sudden death with an interarterial course of an anomalous coronary artery include cyclical compression between the aorta and pulmonary artery, stretching of the intramural segment, or kinking of its origin due to an abnormal acute angulation of the takeoff from the aorta. Furthermore, when the coronary artery arises anomalously off of the aorta, the orifice is often slit-like and more easily compressed by the muscular wall of the aorta.
SUGGESTED READING
Dodd JD, Ferencik M, Liberthson RR, et al. Congenital anomalies of coronary artery origin in adults: 34-MDCT appearance. Am J Roentgenol 2007;188:W138–W146.
Kini S, Kostaki GB, Weaver L. Normal and variant coronary arterial and venous anatomy on high-resolution CT angiography. Am J Roentgenol 2007;188:1665–1674.
GLEN A.
TOOMAYAN
HISTORY
A 66-year-old asymptomatic obese male with a recent positive exercise treadmill stress test performed as part of a comprehensive physical examination. Subsequent stress-rest myocardial perfusion scintigraphy demonstrated inferior wall ischemia. Coronary CTA was performed for further evaluation, as the referring cardiologist believed the scintigraphic findings may represent a false-positive result.
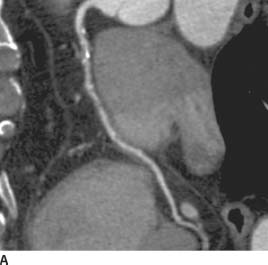
 FIGURE 11-7A Curved multiplanar reformatted (MPR) image of the RCA from a coronary CT angiogram (CTA) demonstrates several calcified and noncalcified plaques in the proximal RCA, which cause mild (<25%) narrowing of luminal diameter. In the mid-RCA, there is an eccentric noncalcified plaque causing significant (>50%) narrowing of the vessel diameter.
FIGURE 11-7A Curved multiplanar reformatted (MPR) image of the RCA from a coronary CT angiogram (CTA) demonstrates several calcified and noncalcified plaques in the proximal RCA, which cause mild (<25%) narrowing of luminal diameter. In the mid-RCA, there is an eccentric noncalcified plaque causing significant (>50%) narrowing of the vessel diameter.

 FIGURE 11-7B Straight MPR image of the RCA from the same examination demonstrates this complex soft plaque in the mid-RCA just distal to a coarse calcified plaque (left is proximal, right is distal). There is significant (>50%) focal narrowing of the luminal diameter at the site of this noncalcified plaque (between long lines) when compared to the normal caliber RCA distally. Several <25% stenoses are seen proximally and distally.
FIGURE 11-7B Straight MPR image of the RCA from the same examination demonstrates this complex soft plaque in the mid-RCA just distal to a coarse calcified plaque (left is proximal, right is distal). There is significant (>50%) focal narrowing of the luminal diameter at the site of this noncalcified plaque (between long lines) when compared to the normal caliber RCA distally. Several <25% stenoses are seen proximally and distally.
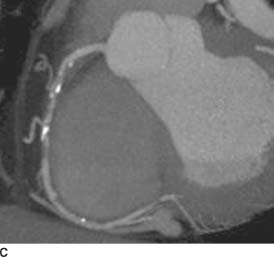
 FIGURE 11-7C Maximum intensity projection (MIP) image of the right coronary artery from the same examination demonstrates the same eccentric non-calcified plaque (just distal to the coarse calcification), which causes a focal >50% stenosis.
FIGURE 11-7C Maximum intensity projection (MIP) image of the right coronary artery from the same examination demonstrates the same eccentric non-calcified plaque (just distal to the coarse calcification), which causes a focal >50% stenosis.
 Atherosclerotic stenosis of the right coronary artery (RCA): Coronary CTA demonstrated multiple eccentric calcified and noncalcified plaques within the RCA. A noncalcified plaque in the mid-RCA caused a >50% narrowing of the luminal diameter. The patient was therefore referred to coronary artery angiography, which revealed a stenosis of 90% in the mid-RCA. This lesion was treated with balloon angioplasty and stenting.
Atherosclerotic stenosis of the right coronary artery (RCA): Coronary CTA demonstrated multiple eccentric calcified and noncalcified plaques within the RCA. A noncalcified plaque in the mid-RCA caused a >50% narrowing of the luminal diameter. The patient was therefore referred to coronary artery angiography, which revealed a stenosis of 90% in the mid-RCA. This lesion was treated with balloon angioplasty and stenting.
DIAGNOSIS
Hemodynamically significant atherosclerotic stenosis of the right coronary artery
KEY FACTS
Clinical
 Coronary artery disease (CAD) is the leading cause of morbidity and mortality in the United States.
Coronary artery disease (CAD) is the leading cause of morbidity and mortality in the United States.
 The prevalence of CAD in the United States is 16 million.
The prevalence of CAD in the United States is 16 million.
 1.2 million new cases of CAD are diagnosed annually in the United States.
1.2 million new cases of CAD are diagnosed annually in the United States.
 Annually, 450,000 deaths are attributed to CAD in the United States.
Annually, 450,000 deaths are attributed to CAD in the United States.
 Symptomatic CAD manifests as angina, heart failure, or acute coronary syndrome. Due to the potential need for intervention, patients with high suspicion for atherosclerotic CAD are directed to coronary angiography.
Symptomatic CAD manifests as angina, heart failure, or acute coronary syndrome. Due to the potential need for intervention, patients with high suspicion for atherosclerotic CAD are directed to coronary angiography.
 Roles for noninvasive imaging of the coronary arteries are currently being defined.
Roles for noninvasive imaging of the coronary arteries are currently being defined.
 Coronary CTA has a high negative predictive value (95% to100%). Therefore, one of its greatest strengths is in excluding coronary artery stenosis in low-to-intermediate risk patients.
Coronary CTA has a high negative predictive value (95% to100%). Therefore, one of its greatest strengths is in excluding coronary artery stenosis in low-to-intermediate risk patients.
 In comparison with conventional coronary angiography, 16- and 64-slice CTA has a sensitivity of 83% to 99% and a specificity of 93% to 98% for the detection of hemodynamically significant coronary artery stenosis (>50% luminal narrowing) in arteries with a diameter >1.5 to 2.0 mm.
In comparison with conventional coronary angiography, 16- and 64-slice CTA has a sensitivity of 83% to 99% and a specificity of 93% to 98% for the detection of hemodynamically significant coronary artery stenosis (>50% luminal narrowing) in arteries with a diameter >1.5 to 2.0 mm.
 When clinical distinction must be made between atherosclerotic and nonatherosclerotic CAD, coronary CTA can demonstrate causes of nonatherosclerotic CAD including coronary artery anomalies, myocardial bridges, and coronary artery aneurysms.
When clinical distinction must be made between atherosclerotic and nonatherosclerotic CAD, coronary CTA can demonstrate causes of nonatherosclerotic CAD including coronary artery anomalies, myocardial bridges, and coronary artery aneurysms.
 Coronary CTA may be useful in assessing a patient’s CAD risk by quantification of calcified and soft plaque.
Coronary CTA may be useful in assessing a patient’s CAD risk by quantification of calcified and soft plaque.
 Coronary CTA may one day be used to monitor response of CAD to pharmacological treatment by monitoring changes in plaque burden.
Coronary CTA may one day be used to monitor response of CAD to pharmacological treatment by monitoring changes in plaque burden.
Radiologic
 Recent technological advances have made noninvasive imaging of the coronary arteries possible.
Recent technological advances have made noninvasive imaging of the coronary arteries possible.
 The introduction of 16- and more recently 64-slice CT scanners and dual source CT scanners has led to improved temporal and spatial resolution, fewer motion artifacts, and decreased reliance on patient cooperation.
The introduction of 16- and more recently 64-slice CT scanners and dual source CT scanners has led to improved temporal and spatial resolution, fewer motion artifacts, and decreased reliance on patient cooperation.
 Imaging the heart during diastole minimizes cardiac motion. With 64-slice CT, lower heart rates are preferable to lengthen the optimal imaging window during diastole. Beta-blockers may be administered to lower the heart rate.
Imaging the heart during diastole minimizes cardiac motion. With 64-slice CT, lower heart rates are preferable to lengthen the optimal imaging window during diastole. Beta-blockers may be administered to lower the heart rate.
 Imaging may be acquired with either prospective ECG triggering or retrospective ECG gating.
Imaging may be acquired with either prospective ECG triggering or retrospective ECG gating.
 Prospective triggering monitors the ECG and triggers image acquisition only during a predefined portion of the cardiac cycle (diastole). Images are not acquired during systole, resulting in a lower radiation dose to the patient compared with retrospective gating.
Prospective triggering monitors the ECG and triggers image acquisition only during a predefined portion of the cardiac cycle (diastole). Images are not acquired during systole, resulting in a lower radiation dose to the patient compared with retrospective gating.
 Retrospective gating acquires continuous images of the heart and records the patient’s ECG simultaneously. Images during diastole are then reconstructed retrospectively by analyzing the ECG. Patient dose is higher with retrospective gating due to continuous image acquisition throughout the cardiac cycle.
Retrospective gating acquires continuous images of the heart and records the patient’s ECG simultaneously. Images during diastole are then reconstructed retrospectively by analyzing the ECG. Patient dose is higher with retrospective gating due to continuous image acquisition throughout the cardiac cycle.
 Patient radiation dose from coronary CTA can be as high as 8 to 22 mSv with retrospective EKG gating and is lower with prospective EKG gating, usually in the order of 2 to 4 mSV. Radiation dose from conventional coronary catheter angiography ranges from 2 to 10 mSV.
Patient radiation dose from coronary CTA can be as high as 8 to 22 mSv with retrospective EKG gating and is lower with prospective EKG gating, usually in the order of 2 to 4 mSV. Radiation dose from conventional coronary catheter angiography ranges from 2 to 10 mSV.
 Accurate evaluation of coronary arteries for significant (50%) luminal narrowing requires software capable of generating multiplanar, straight, and curved reformations.
Accurate evaluation of coronary arteries for significant (50%) luminal narrowing requires software capable of generating multiplanar, straight, and curved reformations.
 Coronary CTA may be used to divide patients into three different groups: those with no evidence of coronary atherosclerotic disease, those with atherosclerotic plaque that results in luminal narrowing of >50%, and those with plaque that causes a luminal stenosis of >50%.
Coronary CTA may be used to divide patients into three different groups: those with no evidence of coronary atherosclerotic disease, those with atherosclerotic plaque that results in luminal narrowing of >50%, and those with plaque that causes a luminal stenosis of >50%.
 Patients with coronary stenosis >50% on coronary CTA may be further evaluated with functional stress testing or conventional catheter angiography.
Patients with coronary stenosis >50% on coronary CTA may be further evaluated with functional stress testing or conventional catheter angiography.
 Limitations of coronary CTA include difficulty imaging patients with rapid heart rates and cardiac arrhythmias, streak artifact from extensive calcified plaque or coronary artery stents, and inability to intervene at the time of diagnosis.
Limitations of coronary CTA include difficulty imaging patients with rapid heart rates and cardiac arrhythmias, streak artifact from extensive calcified plaque or coronary artery stents, and inability to intervene at the time of diagnosis.
SUGGESTED READING
Cody DD, Mahesh M. AAPM/RSNA physics tutorial for residents: Technologic advances in multidetector CT with a focus on cardiac imaging. Radiographics 2007;27:1829–1837.
Achenbach S. Computed tomography coronary angiography. J Am Coll Cardiol 2006;48:1919–1928.
Gaspar T, Halon D, Rubinshtein R, et al. Clinical applications and future trends in cardiac CTA. Eur Radiol 2005;15(Suppl 4):D10-D14.
Schoenhagen P, Halliburton SS, Stillman AE, et al. Noninvasive imaging of coronary arteries: current and future role of multi-detector row CT. Radiology 2004;232:7-17.
Schoepf UJ, Zwerner PL, Savino G, et al. How I do it: Coronary CT angiography. Radiology 2007:244:48-63.
NICOLE PROSCIA
HISTORY
A 63-year-old female with hypertension and hyperlipidemia presents with chest pain.
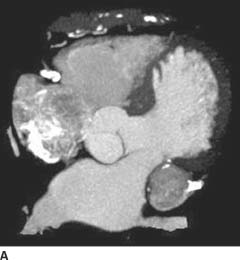
 FIGURE 11-8A MIP axial image from a coronary CT angiogram (CTA) demonstrates a heterogeneously enhancing round mass with peripheral calcification in the left atrioventricular (AV) groove. Note marked calcification of the right coronary artery in the right AV groove.
FIGURE 11-8A MIP axial image from a coronary CT angiogram (CTA) demonstrates a heterogeneously enhancing round mass with peripheral calcification in the left atrioventricular (AV) groove. Note marked calcification of the right coronary artery in the right AV groove.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


