■
Introduction
Cardiac MRI is a study often used for the evaluation and diagnosis of a wide range of acquired and congenital cardiac abnormalities. Cardiac MRI offers excellent soft tissue contrast that is useful in evaluating cardiac masses and infiltrative diseases of the myocardium. Both ECG and respiratory gating improve temporal resolution, allowing for the detection of cardiac motion abnormalities and evaluation of small structures adjacent to the contracting myocardium, such as the coronary arteries. In addition to the anatomic information provided by cardiac MRI, ventricular function and blood flow can be calculated using cine sequences and velocity-encoded cine (VEC) phase contrast imaging.
■
Basic Cardiac MRI
MRI Pulse Sequences
There are two broad categories of MRI sequences used in cardiac MRI: black blood imaging and bright blood imaging. Black (dark) blood imaging is predominantly used to evaluate anatomy and characterize the T1 and T2 properties of tissue. The sequence is referred to as black blood because double-inversion pulse, fast-flowing blood in the imaged slice appears black. Black blood images can be obtained with spin-echo (SE), turbo spin-echo (TSE), or fast spin-echo (FSE) pulse sequences, with echo and repetition times chosen to create a T1- or T2-weighted image.
Bright blood imaging can be used to evaluate both anatomy and motion, usually myocardial contraction, cardiac valve function, and visualization of stenotic or regurgitant flow jets. The sequence is referred to as bright blood because, in contrast to black blood images, the blood pool is brighter than the myocardium. Bright blood images are usually obtained with balanced steady-state free precession (bSSFP) but can also be obtained with spoiled steady-state free precession (sSSFP).
VEC phase contrast imaging is used to measure the velocity of blood throughout the cardiac cycle. This is usually applied to the aorta and pulmonary arteries, where velocity measurements can be used to calculate net forward flow, regurgitant fraction, peak velocity, and pressure gradient. The pulmonary to systemic blood flow ratio (Qp:Qs) can be calculated using VEC MRI to look for evidence of shunts.
Delayed contrast enhancement (DCE) imaging is used to detect any cause of an expanded extravascular space, such as myocardial fibrosis in the setting of ischemia and cardiomyopathy or myocyte injury in myocarditis. DCE can also be used to determine if a myocardial mass has a blood supply, which can differentiate thrombus from tumor.
Imaging Planes
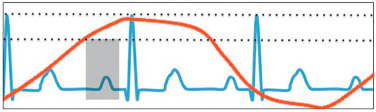
The standard cardiac planes are short-axis, four-chamber (horizontal long axis), and vertical long-axis (two-chamber) views. Additional commonly used cardiac planes include the right ventricular outflow tract, left ventricular outflow tract, right horizontal long axis, and an oblique sagittal used for the evaluation of the aortic arch and descending aorta. These planes are illustrated in Fig. 9.1 . In respiratory gating, imaging is only performed when the diaphragm is in an acceptable position and the heart is in diastole ( Fig. 9.2 ). In this example, imaging was only possible during the first diastole (gray box), ECG signal (blue line), diaphragm displacement (red line), and acceptable diaphragm position (dotted lines).
ECG and Respiratory Gating
ECG gating makes it possible to create cine video clips of cardiac motion throughout the entire cardiac cycle. Alternatively, images can be obtained during preselected segments of the cardiac cycle, most commonly during the R-R interval corresponding to late diastole, when the least amount of cardiac motion is present. Usually, patients are required to hold their breath during the scan.
Respiratory gating is used to compensate for respiratory motion when the patient is breathing freely (see Fig. 9.2 ). The scanner can use the MR signal to monitor the position of the diaphragm and image only when the diaphragm is in an acceptable location. An alternative approach to respiratory gating uses respiratory bellows, which are devices encircling the abdomen and chest that correlate body wall motion with the phase of respiration.
■
Common Indications for Cardiac MRI
Ischemic Heart Disease
Cardiac MRI plays an important role in evaluating both acute and chronic myocardial ischemia. Cardiac MRI can be used to evaluate for myocardial edema, myocardial infarction (MI), and wall motion abnormalities. An important characteristic of ischemia is the distribution of the abnormal findings, which should conform to the coronary artery vascular distributions and, in the case of fibrosis, should be subendocardial.
Myocardial edema appears as high signal on T2-weighted black blood sequences. An abnormally high T2 signal can be difficult to perceive; it has been defined as signal that is two standard deviations (SDs) higher than in remote, presumably normal, myocardium. Alternatively, an abnormal T2 signal is defined as two SDs greater than signal in skeletal muscle on the same slice. In the setting of acute MI, the myocardium that has a high T2 signal but no late gadolinium enhancement (LGE) is at risk for future infarction if the blood flow is not restored.
An MI begins in the endocardium and progressively extends outward, toward the epicardium. The pattern of LGE reflects this physiology, with enhancement beginning in the subendocardium and progressing toward the epicardium. As ischemia persists, there is eventual transmural enhancement. The larger the percentage of myocardial wall thickness demonstrating LGE, the less likely that function will be recovered if the patient is revascularized. It is commonly accepted that the myocardium will be unlikely to recover function if more than 50% of its wall thickness shows LGE.
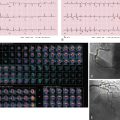
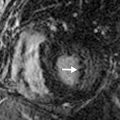
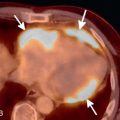
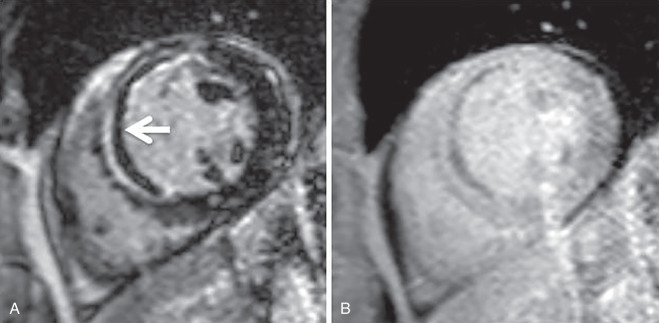
Another imaging feature associated with nonviable myocardium is microvascular obstruction. This occurs in cases of severe MI, in which myocardial capillaries are obstructed due to cellular debris from myocardial necrosis and vessel damage. Because of the microvascular obstruction, gadolinium (Gd) contrast agent cannot penetrate these areas, and they appear dark on LGE images. The imaging hallmark is a dark endocardial region, commonly referred to as a no-reflow zone, surrounded by bright myocardium, which is infarcted ( Fig. 9.3 ).
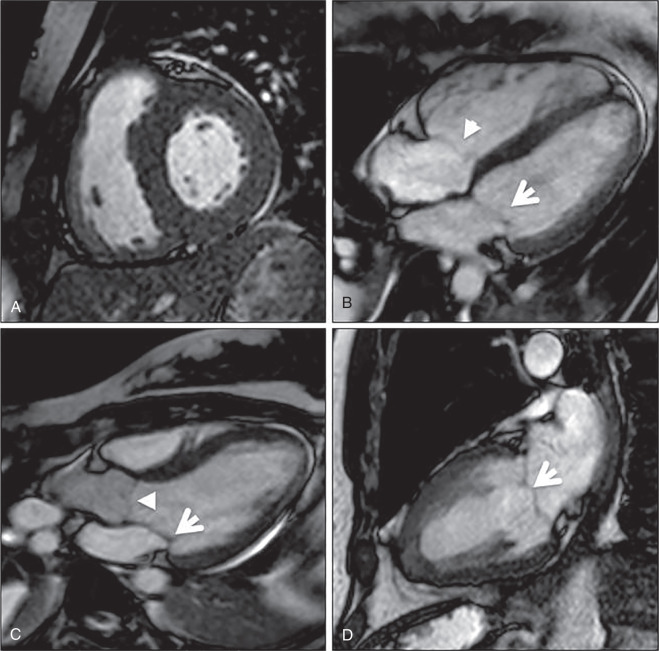
Wall motion abnormalities are associated with MI and, when they are present in the absence of LGE, they may reflect hibernating myocardium that will recover function following revascularization. Wall motion is reported as normal, hyperkinetic, akinetic, or dyskinetic. Location of the abnormality is commonly reported using the 17-segment heart model nomenclature. Wall motion is best assessed with bright blood cine imaging ( Fig. 9.4 ). A stack of short-axis images can be inspected for both wall motion and, more importantly, at least 50% wall thickening from diastole to systole. Some centers will routinely apply grid tags as a prepulse sequence so that strain can be measured. More recently, strain has been measured using feature tracking, without the need for tagging.
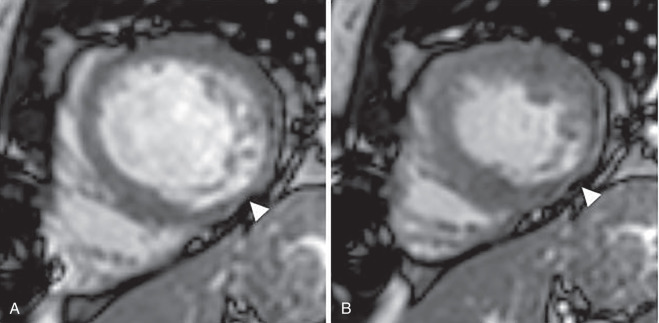
Cardiac MRI also plays a role in imaging chronic myocardial ischemia. In addition to the findings of myocardial ischemia described above, the presence of myocardial thinning suggests that the ischemia is long-standing. Patients with a hibernating myocardium can be identified as having a wall motion abnormality, with no LGE. These patients are likely to recover function if they are revascularized.
Cardiomyopathy
Cardiac MRI is used to diagnose and evaluate the functional impact of cardiomyopathy (e.g., hypertrophic, arrhythmogenic right ventricular dysplasia [ARVD]). A wide variety of conditions fall into the category of what is referred to as cardiomyopathy, which is a diagnosis of myocardial disease with associated cardiac dysfunction. The types of cardiomyopathy most often imaged and those with characteristic cardiac MRI findings include (1) hypertrophic cardiomyopathy (HCM); (2) ARVD; (3) dilated cardiomyopathy; (4) sarcoidosis; and (5) amyloidosis.
HCM is an autosomal dominant disease of incomplete penetrance. The imaging diagnosis is based predominantly on a left ventricular (LV) wall thickness, 15 mm or more, measured at end-diastole. A septal–to–lateral wall ratio greater than 1.3 is an often-discussed criterion for diagnosis but is not as widely accepted.
Cardiac MRI also provides additional information to assist in risk stratification and determine the treatment plan. HCM patients have an increased risk of arrhythmia if there is LGE in areas of thickened myocardium. A myocardial thickness greater than 30 mm is associated with an increased risk of sudden cardiac death. An LV mass can be calculated using cardiac MRI and is associated with overall risk. HCM has several morphologic subtypes described by the distribution of hypertrophy, including septal, midventricular, apical, and symmetric. Cardiac MRI provides an excellent delineation of myocardial hypertrophy distribution when compared to echocardiography. The septal distribution of HCM can often cause a subaortic stenosis that results in systolic anterior motion (SAM) of the mitral valve leaflet. This effect can be exacerbated by exercise.
ARVD is a progressive disease in which the myocardium is replaced by fibrofatty tissue over time. ARVD is associated with ventricular arrhythmias and sudden cardiac death. Patients are typically diagnosed in adulthood based on a combination of major and minor criteria encompassing imaging characteristics, ECG findings, histopathology, and family history. Cardiac MRI findings are included as major and minor criteria in the diagnostic criteria for ARVD. A regional right ventricular (RV) wall motion abnormality, in combination with a decreased RV ejection fraction or abnormal ratio of RV end-diastolic volume relative to body surface area, can fulfill major or minor criteria, depending on the severity of the abnormality. Although detection of fat within the myocardium using cardiac MRI is often discussed, this is no longer a criterion for diagnosis. More commonly seen is RV dilation with regional wall motion abnormalities. Additionally, aneurysms of the RV wall can be seen in ARVD. Abnormal fibrofatty tissue may also be present in the LV myocardium, not only the RV wall.
Dilated cardiomyopathy is often described as ischemic or nonischemic in regard to cause. As described above, ischemic cardiomyopathy is due to coronary artery disease and myocardial ischemia, resulting in thinning and poor contractility of the myocardium. Nonischemic cardiomyopathy has a number of causes, including familial forms of cardiomyopathy, myocarditis, alcoholism, toxins, and cardiomyopathy in pregnancy. Due to the dilation of the ventricular chambers and frequently poor contractility of the myocardium, dilated cardiomyopathy is typically associated with impaired systolic function. Calculation of end-diastolic and end-systolic ventricular volumes is of paramount importance in these patients, who often undergo serial evaluations to monitor ventricular dilation and ejection fraction. It is especially important in these patients to maintain a consistent method of calculating cardiac function.
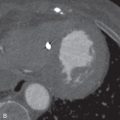
Cardiac amyloidosis is characterized by the extracellular deposition of glycoproteins (amyloid) within the myocardium. On cardiac MRI, the myocardium is diffusely thickened and hypokinetic. Typically, amyloid deposition in the myocardium delays clearance of Gd, which leads to globally increased T1 relaxation. Therefore, the LV MR signal nulls at the same rate as the blood pool. Another pattern seen with LGE is circumferential subendocardial enhancement. Amyloid can present with patchy LGE that is neither confined to a coronary artery distribution nor exclusively subendocardial ( Fig. 9.5 ).