Cardiomyopathy
QUESTIONS
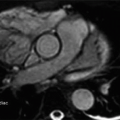
1 Which of the following is a major/minor criterion for arrhythmogenic right ventricular dysplasia?
A. Normalized RV end-diastolic volume of 90 mL/m2
B. Fatty infiltration of the ventricular wall
C. Right ventricular ejection fraction of 50%
D. Dyssynchronous RV wall motion
View Answer
1 Answer D. Arrhythmogenic right ventricular dysplasia (ARVD) is a genetic cardiomyopathy with an incidence of the familial form of ARVD that is estimated between 15% and 50%. ARVD is difficult to diagnose.
The diagnosis is based on clinical and nonclinical criteria, including family history, which are subdivided into major and minor criteria. A “definite” diagnosis consists of two major criteria or one major and two minor criteria, or else four minor criteria. A “borderline” diagnosis consists of one major and one minor criterion or three minor criteria. Finally, a “possible” diagnosis consists of one major or two minor criteria.
The original criteria were revised in 2010 to include specific abnormalities that may be detected on MRI and echocardiography. Primary imaging criteria on MRI include a combination of
Regional RV akinesia, dyskinesia, or dyssynchronous RV contraction
Findings of right ventricular dysfunction, of which severity determines whether a major or minor criteria is reached:
RV end-diastolic volume index ≥110 mL/m2 (male) or ≥100 mL/m2 (female)
Or RV ejection fraction ≤40%, which in combination with (a) would fulfill a Major criteria
100 ≤ RV end-diastolic volume (EDV) < 110 mL/m2 (male) or 90 ≤ EDV < 100 mL/m2 (female)
Or 40% < RV ejection fraction ≤ 45%, which in combination with a) would fulfill a Major criteria
Other morphologic abnormalities such as fat infiltration and delayed enhancement images are detectable by MRI, but have not been considered sensitive enough to be considered in the diagnostic criteria.
References: Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia proposed modification of the task force criteria. Circulation 2010;121(13):1533-1541.
Tavano A, Maurel B, Gaubert J-Y, et al. MR imaging of arrhythmogenic right ventricular dysplasia: what the radiologist needs to know. Diagn Interv Imaging 2015;96(5):449-460.
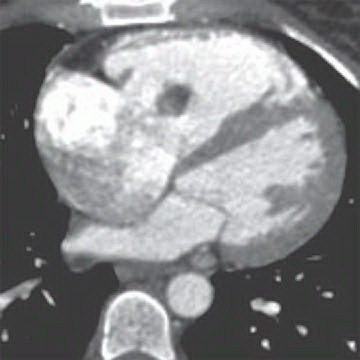
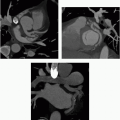
2 A 26-year-old patient with fever, weight loss, and blood cultures positive for Staphylococcus infection.
|
Given the history and imaging findings, which is the most likely etiology?
A. HOCM
B. Myxomatous mitral valve
C. Paradoxical embolus
D. Infective endocarditis
View Answer
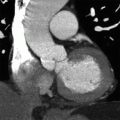
2 Answer D. Contrast-enhanced CT demonstrates a large vegetation on the septal tricuspid leaflet consistent with infective endocarditis (IE).
The risk for IE among IV drug abusers is severalfold greater than that for patients with rheumatic heart disease or prosthetic valves. 65% to 80% of such cases of IE occur in men aged 27 to 37 years. IE is located on the tricuspid valve in 46% to 78%, mitral valve in 24% to 32%, and aortic valve in 8% to 19%; as many as 16% of patients have infection at multiple sites.
IE is a rare complication of hypertrophic cardiomyopathy. It is estimated that incidence is 1.4 per 1,000 person/year in all patients and it increases to 3.8 per 1,000 person/year in patients with left ventricular outflow obstruction and left atrial enlargement.
Mitral valve prolapse (MVP) is the most common cause of isolated mitral regurgitation requiring surgical treatment in the United States and the most common cardiac condition predisposing patients to infective endocarditis. However, the frequency of mitral valve prolapse in IE is not entirely a direct reflection of relative risk but rather a function of the frequency of the lesion in the general population. Risk factors for infective endocarditis in patients with MVP include the presence of mitral regurgitation or thickened mitral leaflets and account for 7% to 30% of native-valve endocarditis not related to drug abuse or nosocomial infection.
Risk of infective endarteritis in patients with patent ductus arteriosus seems to have declined during the last 30 to 40 years, and cases of patent ductus arteriosus complicated by infective endarteritis are now very rare.
References: Karchmer CM. Infectious endocarditis. In: Bonow RO, Braunwald E (eds). Braunwald’s heart disease: A textbook of cardiovascular medicine. Philadelphia, PA: Saunders, 2012:1540-1560.
Louahabi T, Drighil A, Habbal R, et al. Infective endocarditis complicating hypertrophic obstructive cardiomyopathy. Eur Heart J Cardiovas Imaging 2006;7(6):468-470.
Sadiq M, Latif F, ur-Rehman A. Analysis of infective endarteritis in patent ductus arteriosus. Am J Cardiol 2004;93(4):513-515.
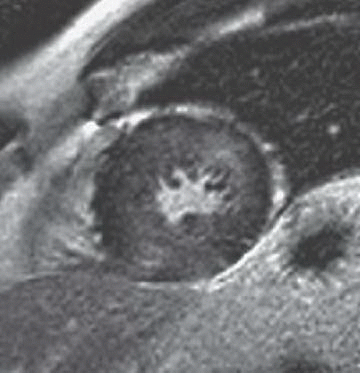
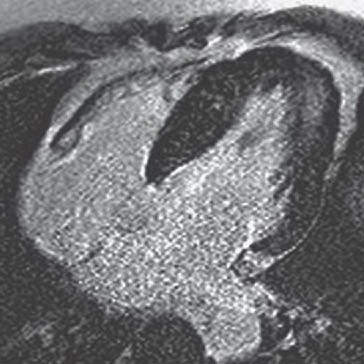
A. Myocardial infarction
B. Hypertrophic cardiomyopathy
C. Myocarditis
D. Arrhythmogenic right ventricular dysplasia
View Answer
3 Answer B. Short-axis view of the heart demonstrating marked hypertrophy of the left ventricle. The MR sequence is an inversion recovery technique with an inversion time to null the myocardium and assess for late gadolinium enhancement. In this case, there is patchy enhancement within the midmyocardium with more focal fibrosis in anterolateral and inferoseptal segments. Findings are consistent with hypertrophic cardiomyopathy.
The pattern of enhancement is atypical for infarction, which should primarily be subendocardial. Left ventricular hypertrophy and patchy enhancement can be seen with amyloidosis. However, the nulling of the myocardium is characteristically difficult because of the T1 properties of the amyloid protein and diffusely increased extracellular volume.
Hypertrophic cardiomyopathy (HCM) is a genetic disorder characterized by left ventricular hypertrophy (wall thickness >12 to 15 mm) but is also heterogeneous in presentation, prognosis, and treatment strategies. Pathologic hallmarks of HCM include myocyte disarray and interstitial fibrosis.
Several recognized imaging phenotypes are recognized (e.g., asymmetric [septal] HCM, apical HCM, symmetric HCM [concentric HCM], and midventricular HCM). Increased LV wall thickness may result in narrowing of the left ventricular outflow tract (LVOT). Systolic anterior motion of the anterior mitral leaflet may also be observed, which can increase LVOT obstruction and decreased coronary and systemic outflow.
LV wall thickness, presence of underlying perfusion abnormalities, and fibrosis as evidenced by late gadolinium enhancement are important imaging markers pointing to increased risk for sudden death in HCM patients.
References: Hoey ETD, Teoh JK, Das I, et al. The emerging role of cardiovascular MRI for risk stratification in hypertrophic cardiomyopathy. Clin Radiol 2014;69(3):221-230.
Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002;287(10):1308-1320.
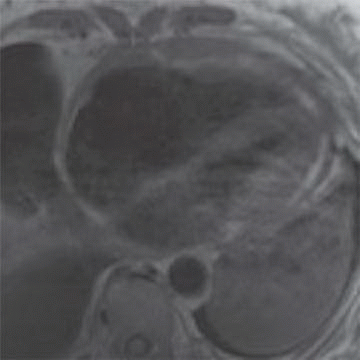
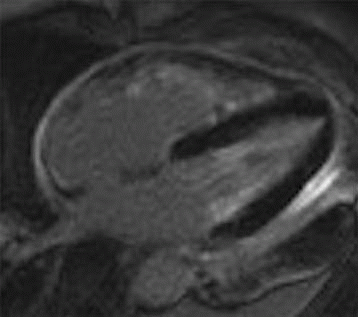
A. Restrictive cardiomyopathy
B. Constrictive pericarditis
C. Hypertrophic cardiomyopathy
D. Left ventricular noncompaction
View Answer
4 Answer B. T1-weighted axial image demonstrates significant near-circumferential thickening of the pericardium (in this case, measuring >1 cm in max thickness). Findings are consistent with constrictive pericarditis.
Constrictive pericarditis (CP) is characterized by fibrous or calcific thickening of the pericardium, which prevents normal diastolic filling of the heart. Historically, tuberculosis was the most common cause of CP and was frequently associated with extensive pericardial calcification. In the modern era, tuberculous pericarditis is rare, and important causes are increasingly previous mediastinal irradiation and cardiac surgery.
Patients commonly present with signs and symptoms of right-sided heart failure that are disproportionate to the severity of left ventricular dysfunction or valvular disease. Both restrictive and constrictive diseases exhibit an abrupt reduction in filling, increased backpressure, and impaired stroke volume. It is important to distinguish between constrictive pericarditis and restrictive cardiomyopathy because treatment for the former condition is surgical, and treatment for the latter is medical.
Classically, the pericardium in patients with CP will be diffusely thicker than 4 mm; however, the diagnosis of should not be completely disregarded if thickening is not present. Cardiac chambers will be within normal limits in size. Superior and inferior vena cava as well as hepatic veins may be dilated. The influence of respiration on filling contributes important diagnostic information, both for imaging and for catheterization. The restriction in CP creates discordance with reduced left ventricular filling, which corresponds to increased right ventricular filling. This manifests as a variance in the septal curvature during diastolic filling and a characteristic “septal bounce.”
Restrictive cardiomyopathy is characterized by a marked decrease in ventricular compliance and results from a number of etiologies, including hypertrophic cardiomyopathy. Imaging often reveals thickening of the ventricles with biatrial enlargement.
Left ventricular noncompaction (VNC) is a congenital myocardial abnormality that can present in either childhood or adulthood with congestive heart failure, arrhythmia, or thromboembolism. VNC occurs due to persistence of noncompacted endocardium characteristic of the early fetal period before myocardial compaction is complete. The left ventricle is usually affected, and it may be either dilated or hypertrophied.
References: Hughes S. Cardiomyopathies. In: Suvarna SK (ed.). Cardiac pathology. London, UK: Springer, 2013:183-200.
Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999;100(13):1380-1386.
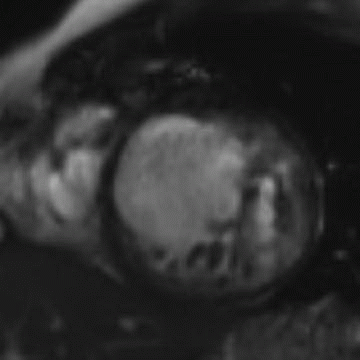
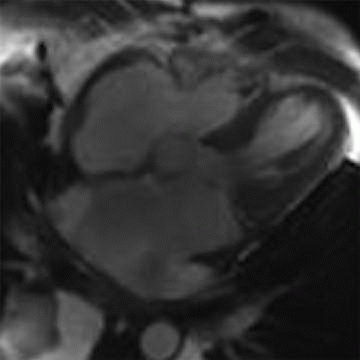
5 Patient is a 26-year-old with worsening heart failure. Given the imaging findings, what is the most likely explanation for the patient’s condition?
|
A. Restrictive cardiomyopathy
B. Constrictive pericarditis
C. Hypertrophic cardiomyopathy
D. Left ventricular noncompaction
View Answer
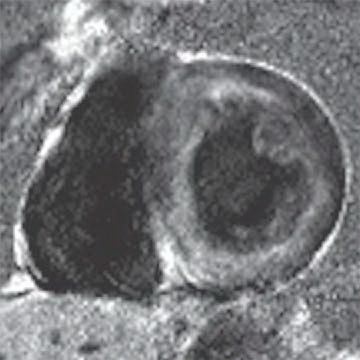
5 Answer D. Midventricular short-axis SSFP image of the heart demonstrates significant increased trabeculation of the left ventricular cavity relative to normal compacted myocardium. This appearance is compatible with left ventricular noncompaction (VNC). Note that the papillary muscles are often not well formed in the setting of VNC.
Left ventricular noncompaction, also known as spongiform cardiomyopathy, is a congenital myocardial abnormality that can present in either childhood or adulthood. VNC occurs due to persistence of noncompacted endocardium characteristic of the early fetal period before myocardial compaction is complete. The left ventricle is usually affected, and it may be either dilated or hypertrophied.
Echocardiography or MRI is the diagnostic method of choice for detecting VNC cardiomyopathy and is characterized by prominent trabeculations associated with deep intertrabecular recesses. Diagnostic clues include ≥3 trabeculations in one imaging plane located apically from the insertion of the papillary muscles and a ratio of noncompacted myocardium to compacted myocardium of more than 2.3:1 (sensitivity, 86%; specificity, 99%).
Patients with VNC have high morbidity and mortality as a result of heart failure, ventricular arrhythmias, and systemic embolism. Sudden death by arrhythmia is most often the cause of death. Unfortunately, there is no specific therapy for VNC with the only definitive treatment is cardiac transplant.
References: Petersen SE, Selvanayagam JB, Wiesmann F, et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2005;46(1):101-105.
Zenooz NA, Zahka KG, Siwik ES, et al. Noncompaction syndrome of the myocardium: pathophysiology and imaging pearls. J Thorac Imaging 2010;25(4):326-332.
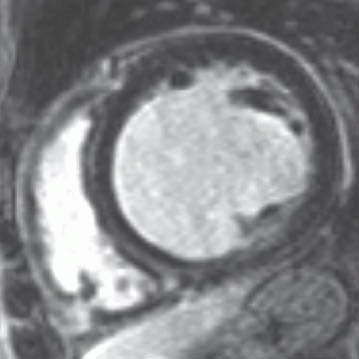
6 Patient is referred for atypical chest pain and exercise intolerance over the last few months. No personal or family history of cardiac disease is otherwise noted. Given the imaging findings, what is the most likely etiology of the patient’s condition?
|
A. Myocardial infarction
B. Hypertrophic cardiomyopathy
C. Myocarditis
D. Arrhythmogenic right ventricular dysplasia
View Answer
6 Answer C. Inversion recovery sequence demonstrating nulling of the myocardium and multifocal areas of late gadolinium enhancement in mid- and epicardial distribution. The findings are most compatible with myocarditis. The epicardial distribution is not compatible with infarction. Hypertrophic cardiomyopathy typically has asymmetric thickening and a familial association. Arrhythmogenic right ventricular cardiomyopathy typically demonstrates abnormality in myocardial nulling.
Myocarditis (inflammatory cardiomyopathy) is inflammation of the heart caused by a variety of pathogens and triggers (e.g., viral, bacterial, fungal infections, drug toxicity, or postradiation). Despite the etiology, the inflammation culminates in leukocytic cell infiltration, nonischemic degeneration, myocyte necrosis, and cardiac dysfunction.
Presence of late gadolinium enhancement (LGE) is an indication of irreversible myocardial necrosis and fibrosis. The pattern is classically subepicardial in distribution, although midinterventricular and focal transmural patterns are also possible. T2-weighted and early gadolinium enhancement imaging techniques are well validated to detect edema and hyperemia, respectively. In addition to LGE, these imaging features comprise the three “Lake Louise Consensus Criteria,” recommended for diagnosing myocarditis. When two or more of these tissue characterization sequences are positive, pooled diagnostic accuracy for myocarditis is 78%; if only delayed enhancement is performed, diagnostic accuracy is 68%.
Quantitative T2 mapping offers the potential for increased accuracy in the detection of myocardial edema. Moreover, T1 mapping promises to overcome the limitation of needing large areas of necrosis to get a sufficient T1 contrast for LGE imaging.
References: Ferreira VM, Piechnik SK, Dall’Armellina E, et al. T1 mapping for the diagnosis of acute myocarditis using CMR. JACC Cardiovas Imaging 2013;6(10):1048-1058.
Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 2009;53(17):1475-1487.
Yilmaz A, Ferreira V, Klingel K, et al. Role of cardiovascular magnetic resonance imaging (CMR) in the diagnosis of acute and chronic myocarditis. Heart Fail Rev 2013;18(6):747-760.
7 Patient is referred for cardiac MR for decreased systolic function and decreased exercise tolerance. Given the imaging findings, what is the most likely cause of the patient’s condition?
|
A. Myocardial infarction
B. Hypertrophic cardiomyopathy
C. Myocarditis
D. Hemochromatosis
View Answer
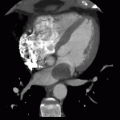
7 Answer D. Inversion recovery sequence on the left demonstrates patchy late gadolinium enhancement consistent with fibrosis. Axial SSFP image on the right shows characteristic dark appearance of the liver compatible with iron deposition and signal loss due to susceptibility. The other etiologies do not demonstrate these changes.
Iron overload cardiomyopathy (IOC) is a secondary form of cardiomyopathy resulting from the accumulation of iron in the myocardium. IOC may result from hereditary disorders of iron metabolism (e.g., cardiomyopathy in hemochromatosis, thalassemia) or may be secondary due to multiple transfusions or abnormalities of hemoglobin synthesis leading to aberrant erythropoiesis.
Two main imaging phenotypes are generally noted—infiltration of the ventricular myocardium resulting in a restrictive cardiomyopathy, common in primary hemochromatosis, and a dilated cardiomyopathy with severe diastolic dysfunction in the early stages of secondary hemochromatosis. Echocardiography may not be able to distinguish IOC from idiopathic dilated cardiomyopathy. Deposition of iron in the myocardium causes a decrease in T2* relaxation time, which can be detected by multiecho gradient sequences on cardiac MRI. T2* values associated with IOC are typically less than 20 msec. The iron load is considered severe if the value is less than 10 msec. Varying amounts of delayed enhancement may also be observed depending on the severity of fibrosis.
References: Anderson L. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J 2001;22(23):2171-2179.
Kremastinos DT, Farmakis D. Iron overload cardiomyopathy in clinical practice. Circulation 2011;124(20):2253-2263.
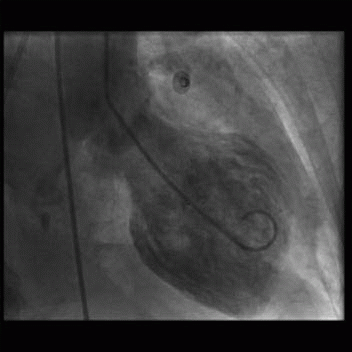
8 A 36-year-old woman presents with acute chest pain and elevated troponins. Apart from mild hypertension, she has no significant past medical history. Emergency cardiac catheterization shows no obstructive coronary artery disease, and the left ventriculogram demonstrates apical hypokinesis at end systole (below).
|
Given the clinical presentation and imaging, which of the following is the most likely etiology?
A. Dilated cardiomyopathy due to alcohol
B. Hypertrophic cardiomyopathy
C. Stress-induced cardiomyopathy
D. Ischemic cardiomyopathy
View Answer
8 Answer C. Given the symptoms of chest pain and elevated troponins, the primary concern would be the presence of an obstructive coronary lesion and need for immediate coronary intervention. The absence of coronary disease and the characteristic appearance of apical dilation on the ventriculogram are compatible with a stress-induced cardiomyopathy, also called Takotsubo cardiomyopathy.
Takotsubo cardiomyopathy (TTC) is a rapidly reversible form of acute heart failure reported to be triggered by stressful events and associated with a distinctive left ventricular (LV) contraction pattern. TTC mimics acute coronary syndrome in clinical presentation in the absence of angiographically significant coronary artery stenosis.
Variants of TTC include apical, midventricular, basal, or biventricular “ballooning”. The most common form is severe anteroapical akinesis and hypercontractility of the basal segments (“apical ballooning”). There is typically an absence of late enhancement on delayed contrast sequences, which differentiates Takotsubo cardiomyopathy from anterior myocardial infarction.
Many studies report a good long-term prognosis; however, the acute phase of TTC can truly be life threatening. Complications, which may occur in the acute setting, include heart failure, arrhythmia, cardiogenic shock, LVOT obstruction, mitral regurgitation, ventricular thrombus, and cardiac rupture.
Treatment of TTC during the acute phase is mainly symptomatic treatment. Patients with TTC usually have a good prognosis, and almost perfect recovery is observed in 96% of the cases.
References: Eitel I, Schuler G, Gutberlet M, et al. Biventricular stress-induced (takotsubo) cardiomyopathy “with left midventricular and right apical ballooning. Int J Cardiol 2011;151(2):e63-e64.
Virani SS, Khan AN, Mendoza CE, et al. Takotsubo cardiomyopathy, or broken-heart syndrome. Tex Heart Inst J 2007;34(1):76-79.
A. Ventricular noncompaction
B. Severe aortic stenosis
C. Uhl anomaly
D. Annuloaortic ectasia
View Answer
9 Answer B. Although commonly seen in the setting of hypertrophic cardiomyopathy, basal septal hypertrophy (BSH) can be seen independently of HCM, being more prevalent in the elderly and in the presence of systemic hypertension. One study identified over 3,500 patients with isolated septal hypertrophy in the Framingham Heart Study, where BSH was characterized as septal thickness greater than 1.4 cm in the absence of septal abnormalities. The presence of BSH was not associated with an increased risk of cardiovascular disease or mortality.
Based on autopsy series and echocardiographic data, about 10% of patients with hemodynamically significant AS show asymmetric thickening of the septum. Recent studies have demonstrated that hypertrophic heart disease from valvular aortic stenosis implicates a poor prognosis early and late after aortic valve replacement. However, it is still controversial whether directed therapy such as myotomy or alcohol ablation should be considered concomitant to aortic valve repair.
Noncompaction, Uhl anomaly and annuloaortic ectasia are not associated with this phenomenon.
References: Di Tommaso L, Stassano P, Mannacio V, et al. Asymmetric septal hypertrophy in patients with severe aortic stenosis: the usefulness of associated septal myectomy. J Thoracic Cardiovas Surg 2013;145(1):171-175.
Diaz T, Pencina MJ, Benjamin EJ, et al. Prevalence, clinical correlates, and prognosis of discrete upper septal thickening on echocardiography: the Framingham Heart Study. Echocardiography 2009;26(3):247-253.
Kelshiker MA, Mayet J, Unsworth B, et al. Basal septal hypertrophy. Curr Cardiol Rev 2013;9(4):316-324.
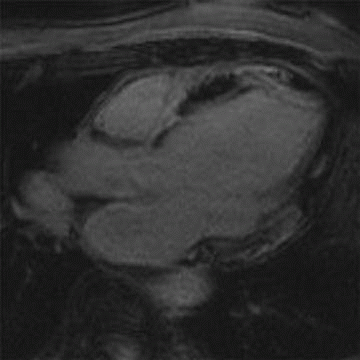
10 A 27-year-old male has a syncopal episode while playing soccer. He was resuscitated by medical personnel in the field and brought to the emergency department. On review of family history, he notes that he had an uncle who unexpectedly died at the same age playing basketball. Cardiac MR demonstrates the following:
|
Given the clinical presentation and the imaging findings, what treatment recommendation would you suggest?
A. Beta blockers
B. ICD
C. ACE inhibitors
D. Steroid therapy
View Answer
10 Answer B. Horizontal long axis demonstrates marked left ventricular thickening with mild asymmetric thickening of the septum, consistent with hypertrophic cardiomyopathy (HCM). Patchy late gadolinium enhancement is also observed near the LV apex, compatible with areas of myocardial fibrosis.
HCM is a clinically and genetically heterogeneous disorder, characterized most commonly by left ventricular (LV) hypertrophy. HCM has a range of potential outcomes including heart failure and sudden cardiac death, but also survival to normal life expectancy.
The estimated prevalence of HCM of 1 in 500 is based on data originally collected almost 20 years ago. However, advances in HCM, including enhanced understanding of the underlying molecular and genetic substrate, contemporary family screening, and more sensitive diagnostic cardiac imaging, suggest that the prevalence of HCM may be underestimated. Although many patients remain asymptomatic with a benign natural history, sudden death (SD) can occur as the initial manifestation of the disease in otherwise asymptomatic or mildly symptomatic young (<25 years of age) patients.
Conventional risk factors for SD in HCM include family history HCM-related sudden death; one of more episodes of unexplained recent syncope; LV hypertrophy >30 mm; nonsustained ventricular tachycardia on 24 hour electrocardiography; and hypotensive blood pressure response to exercise. Cardiac magnetic resonance (CMR) imaging has emerged as a precise diagnostic tool and powerful adjunct in assessing risk of SD with HCM. CMR provides characterization of morphologic phenotypes of LV wall thickening, often not reliably visualized with standard echocardiographic cross-sectional planes. The presence of late gadolinium enhancement (LGE) in HCM patients has been shown to have a sevenfold increased risk for potential lethal ventricular tachyarrhythmias compared with those without LGE. Although large prospective studies validating its utility are still emerging, many experts consider the presence of LGE as influential in the decision of placing an ICD in HCM patients currently classified as intermediate risk.
References: Efthimiadis GK, Pagourelias ED, Gossios T, et al. Hypertrophic cardiomyopathy in 2013: current speculations and future perspectives. World J Cardiol 2014;6(2):26-37.
Hoey ETD, Teoh JK, Das I, et al. The emerging role of cardiovascular MRI for risk stratification in hypertrophic cardiomyopathy. Clin Radiol 2014;69(3):221-230.
Semsarian C, Ingles J, Maron MS, et al. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 2015;65(12):1249-1254.
11 A patient with cardiomyopathy and peripheral eosinophilia presents with the following imaging findings:
|
Which abnormality most likely reflects the underlying cardiomyopathy?
A. Loeffler endocarditis
B. Myocardial infarction
C. Cardiac sarcoidosis
D. Amyloidosis
View Answer
11 Answer A. Horizontal long-axis image of the heart demonstrates subendocardial late gadolinium enhancement involving both ventricles. The remaining myocardium nulls appropriately. Although infarction is classically subendocardial, the distribution of the abnormality does not follow a vascular distribution. The unremarkable nulling of the remaining myocardium is atypical in amyloid. Cardiac sarcoidosis is usually mid- to epicardial in its involvement.
Eosinophil-mediated myocarditis can occur in association with parasitic infection (tropical endomyocardial fibrosis) or Churg-Strauss syndrome (a necrotizing small vessel vasculitis) or can occur as an idiopathic entity (termed Loeffler endocarditis or hypereosinophilic syndrome). Classically, three clinicohistologic stages have been described:
Acute necrotic stage—characterized by subendocardial necrosis as well as constitutional symptoms, pulmonary infiltrates, atrioventricular valve regurgitation, and biventricular failure
Subacute thrombotic stage—characterized by thrombosis, splinter hemorrhages, and more severely resulting in cerebral, splenic, renal, and coronary infarctions
Late fibrotic stage—characterized by late-stage fibrosis of the endomyocardial surface of either or both left and right ventricles
Cardiac MR is instrumental in identifying markers of eosinophilic involvement including myocardial inflammation, mural thrombi, and endocardial fibrosis. The use of first-pass perfusion MRI allows differentiation of perfused and enhancing myocardium from poorly vascularized and hypoenhancing thrombus or eosinophilic infiltrate. Late gadolinium enhancement images typically show intense global subendocardial enhancement that is not limited to a vascular territory. Nonenhancing thrombi may also be observed in left and right ventricular apices.
Endomyocardial fibrosis and especially cardiac thromboembolic events originating from mural thrombus may cause potentially fatal complications or irreversible neurologic defects if not appropriately treated without delay. Follow-up MRI can help document therapeutic improvement with reduction of left ventricular mass and improved contractile function along with simultaneous improvement of clinical symptoms.
References: Kleinfeldt T, Ince H, Nienaber CA. Hypereosinophilic syndrome: a rare case of Loeffler’s endocarditis documented in cardiac MRI. Int J Cardiol 2011;149(1):e30-e32.
Mannelli L, Cherian V, Nayar A, et al. Loeffler’s endocarditis in hypereosinophilic syndrome. Curr Probl Diagn Radiol 2012;41(4):146-148.
Perazzolo Marra M, Thiene G, Rizzo S, et al. Cardiac magnetic resonance features of biopsy-proven endomyocardial diseases. JACC Cardiovas Img 2014;7(3):309-312.
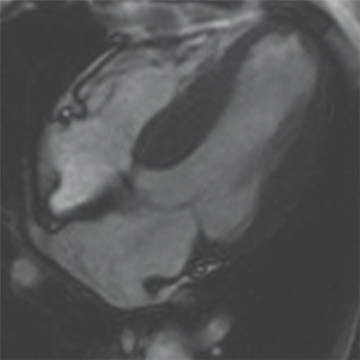
12 A 68-year-old patient with was found to have moderate decreased left ventricular function and severe diastolic dysfunction. Cardiac MR reveals biatrial enlargement, bilateral pleural effusions, and both systolic and diastolic dysfunction (below).
|
Given the clinical picture and imaging findings, which etiology would explain the patient’s condition?
A. Restrictive cardiomyopathy
B. Ischemic cardiomyopathy
C. Dilated cardiomyopathy
D. Inflammatory cardiomyopathy
View Answer
12 Answer A. Horizontal long axis demonstrates biatrial enlargement and relative normal appearance of both ventricles. Whereas both constrictive pericarditis and restrictive cardiomyopathy can present with similar clinical signs, restrictive cardiomyopathy classically demonstrates biatrial enlargement and constrictive pericarditis classically has normal appearance to the cardiac chambers. This results from decreased ventricular compliance. Ventricular enlargement can be seen ischemic, inflammatory, or dilated cardiomyopathies.
Generally, restrictive cardiomyopathy (CMP) refers to a group of primary or secondary infiltrative disorders characterized by normal left ventricular cavity size and systolic function but with increased myocardial stiffness and decreased ventricular compliance. Primary restrictive cardiomyopathies include endomyocardial fibrosis, Loeffler endomyocarditis, and idiopathic primary restrictive CMP. Secondary types of restrictive CMP are more common and are typically due to conditions where the heart is affected as part of a multisystem disorder (e.g., amyloidosis, hemochromatosis).
The morphologic appearance in restrictive CMP often demonstrates atrial enlargement and ventricular thickening. The RV may also enlarge if pulmonary hypertension coexists. Cardiac MRI is a fundamental diagnostic tool because it helps in the differentiation between restrictive CMP and constrictive pericarditis, which have different therapeutic approaches. The presence of late gadolinium enhancement is consistent with fibrosis in myocardium.
Diastolic function is severely disturbed and could easily be assessed by studying the ventricular filling pattern on cine or phase contrast images. The characteristic features of restrictive left ventricular filling are short isovolumic relaxation time, dominant early diastolic filling with short deceleration time, and small or absent late diastolic filling component.
References: Belloni E, De Cobelli F, Esposito A, et al. MRI of cardiomyopathy. Am J Roentgenol 2008;191(6):1702-1710.
Gupta A, Singh Gulati G, Seth S, et al. Cardiac MRI in restrictive cardiomyopathy. Clin Radiol 2012;67(2):95-105.
Hughes S. Cardiomyopathies. In: Suvarna SK (ed.). Cardiac pathology. London: Springer, 2013:183-200.
13 A 47-year-old patient with worsening heart failure presents for evaluation by cardiac MR. A representative image is noted below. Which of the following is most closely associated with the MR finding?
|
A. Nonischemic dilated cardiomyopathy
B. Sarcoid cardiomyopathy
C. Arrhythmogenic right ventricular cardiomyopathy
D. Ischemic cardiomyopathy with septal infarct
View Answer
13 Answer A. Short-axis inversion recovery sequence after contrast demonstrates linear late gadolinium enhancement in the midwall of the interventricular septum.
A linear midwall septal stripe of late gadolinium enhancement has been described in approximately 30% of patients with nonischemic dilated cardiomyopathies. The location of the stripe does not follow a pattern consistent with ischemic disease. The abnormality is thought to develop secondary to replacement fibrosis, which has been reported in pathologic samples and may be related to subclinical foci of myocardial ischemia. This linear abnormality has been suggested as a marker for increased risk of sudden cardiac death, since the fibrosis may predispose to electrical instability.
Reference: Cummings KW, Bhalla S, Javidan-Nejad C, et al. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics 2009;29(1):89-103.
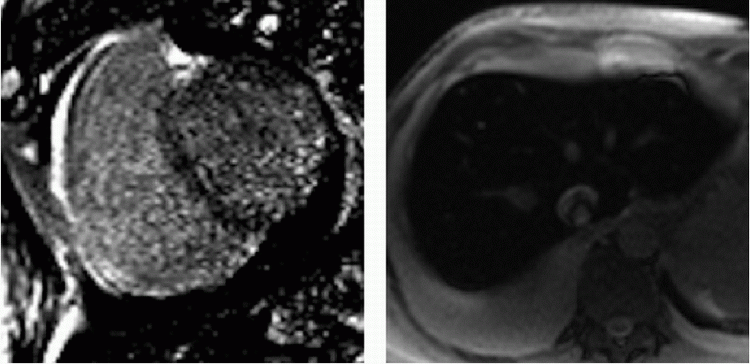
14 A 77-year-old male with multiple myeloma has worsening dyspnea with global ventricular hypertrophy and decreased left ventricular function by echocardiography. Cardiac MR was ordered for further evaluation.
|
Given the clinical history and imaging findings, which of the following diagnoses best characterize the underlying cardiomyopathy?
A. Amyloidosis
B. Dilated cardiomyopathy
C. Hypertrophic cardiomyopathy
D. Iron overload cardiomyopathy
View Answer
14 Answer A. Short-axis image of the left ventricle demonstrates diffusely abnormal nulling of the myocardium on inversion recovery sequences. Classically, with the blood pool containing the largest concentration of gadolinium, the inversion time of the blood pool occurs before nulling of the myocardium. In this case, subendocardial myocardium has traversed its null point before the blood pool, and the remaining myocardium reaches its null point near the same time as the blood pool. This gross aberration of late gadolinium enhancement is almost exclusively seen in the setting of amyloid deposition.
Cardiac amyloidosis is the most common infiltrative type of secondary restrictive cardiomyopathies and is caused by the deposition of insoluble amyloid protein fibrils in the interstitium of the myocardium. Cardiac amyloidosis may be classified according to the type of amyloid fibril protein deposited. The most common type of amyloidosis to affect the heart is AL amyloidosis due to the deposition of amyloid fibrils complexed with monoclonal kappa and lambda immunoglobulin light chains. AL amyloidosis is principally associated with plasma cell dyscrasias (e.g., B-cell lymphoma, Waldenstrom macroglobulinemia, multiple myeloma). Mutations in the gene for transthyretin predominantly result in neurologic and heart disease, and with some mutations, amyloid deposits are exclusive to the myocardium. Fragments of serum amyloid A protein are responsible for AA (secondary) amyloidosis, which is associated with a variety of chronic inflammatory disorders, but rarely associated with cardiac involvement.
No single noninvasive test or abnormality is pathognomonic of cardiac amyloid; diagnosis of cardiac amyloid has usually relied on (1) echocardiographic assessment, especially measurement of LV wall thickness, subjective assessment of myocardial appearance, and evaluation of diastolic function/restrictive physiology, and (2) histopathologic findings of amyloid deposition on endomyocardial biopsy.
The high spatial resolution and signal-to-noise ratio of cardiac MR permit reproducible measurement of cardiac chamber volumes and mass, as well as LV and atrial septal wall thickness. The main feature of cardiac amyloidosis is diffuse myocardial thickening including the atria and valves. Biatrial dilation and restriction of diastolic filling may also be seen associated with depressed systolic ventricular function and reduced wall compliance, which in later stages can evolve to overt restrictive CMP.
Tissue characterization with LGE provides unique clinical value in further assessment of amyloid infiltration. The pattern of late gadolinium enhancement is characterized by a diffuse, heterogeneous subendocardial distribution that may resemble an incorrect myocardial signal suppression due to an inappropriate choice of inversion time.
References: Maceira AM. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2005;111(2):186-193.
Selvanayagam JB, Leong DP. MR imaging and cardiac amyloidosis: “where to go from here? JACC Cardiovas Imaging 2010;3(2):165-167.
15 What is the most common etiology of dilated cardiomyopathies (DCM)?
A. Ischemic
B. Familial
C. Inflammatory
D. Idiopathic
View Answer
15 Answer D. Dilated cardiomyopathies (DCM) are a spectrum of heterogeneous myocardial disorders that are characterized by ventricular dilation and depressed myocardial contractility (typically ejection fraction less than 40%).
The cause is not well understood, and although up to 50% of cases are considered to be idiopathic, it is recognized that other cases of the disease may have ischemic, genetic or familial, viral, immune, or a toxic origin or can be secondary to cardiovascular diseases with myocardial dysfunction that is not explained by ischemic damage. About 20% to 35% cases of idiopathic DCM are familial in origin.
DCM is the most common cause of cardiomyopathy and cardiac transplantation in children and adults. The most common causes of infantile DCM include idiopathic, inborn errors of metabolism, and malformation syndromes. Myocarditis and neuromuscular disorders are the most common causes during childhood.
The most common determination among cardiomyopathies with a dilated phenotype is whether or not the etiology is related to underlying coronary disease (ischemic vs. nonischemic). Existing clinical studies suggest that the prognosis of patients with nonischemic dilated cardiomyopathy is better than patients with underlying ischemic heart disease.
References: Bozkurt B. Chapter 24—heart failure as a consequence of dilated cardiomyopathy. In: Mann DL (ed.) Heart failure: a companion to Braunwald’s heart disease, 2nd ed. Philadelphia, PA: Saunders, 2011:372-394.
Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet 2010;375(9716):752-762.
Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 2006;296(15):1867-1876.
16a A 27-year-old patient presents with atypical chest pain for the last 2 days. Patient is otherwise healthy without significant past medical history. Review of systems reveals recent upper respiratory infection and fever for which he took over-the-counter medication. Echocardiography notes small pericardial effusion but otherwise normal.
Given the clinical picture, which would be the most likely etiology of the patient’s condition?
A. Myocardial infarction
B. Myocarditis
C. Restrictive cardiomyopathy
D. Constrictive pericarditis
View Answer
16a Answer B. Myocarditis (inflammatory cardiomyopathy) is defined as an inflammatory disorder of the myocardium, characterized by leukocytic cell infiltration, nonischemic degeneration, myocyte necrosis, and cardiac dysfunction.
Clinical presentation is variable in severity, ranging from asymptomatic to cardiogenic shock. Myocarditis is typically associated with other viral symptoms, 7 to 10 days after the onset of the systemic illness. Young adults are most commonly affected. The mean age of patients with giant-cell myocarditis (GCM) is 42 years, whereas the mean age of adult patients with other forms of myocarditis has been reported to range from 20 to 51 years.
Currently, no single clinical or imaging finding confirms the diagnosis of myocarditis with absolute certainty. Rather, an integrated synopsis, including history, clinical assessment, and noninvasive test results, should be used to diagnose the disease and guide treatment. Most patients respond well to standard heart failure therapy, although, in severe cases, mechanical circulatory support or heart transplantation is indicated. More than 75% of patients with acute myocarditis gain spontaneous recovery, except in patients with giant-cell myocarditis. Persistent, chronic myocarditis usually has a progressive course but may respond to immunosuppression.
The standard Dallas pathologic criteria for the definition of myocarditis require that an inflammatory cellular infiltrate with or without associated myocyte necrosis be present on conventional endomyocardial biopsy. Noninvasive cardiac magnetic resonance imaging (MRI) may provide an alternative method for diagnosis without the risks of biopsy.
References: Cooper LT Jr. Myocarditis. NEJM 2009;360(15):1526-1538.
Maisch B, Pankuweit S. Current treatment options in (peri)myocarditis and inflammatory cardiomyopathy. Herz 2012;37(6):644-656.
16b Considering the previous question, the MR protocol for the diagnosis includes an inversion recovery sequence for late gadolinium enhancement. What additional MR sequence should be included in the evaluation of myocarditis?
A. Chemical shift imaging
B. Pre- and early postgadolinium enhancement
C. CSPAMM myocardial tagging
D. Contrast-enhanced MR angiography
View Answer




16b Answer B. The expected tissue pathology in active myocarditis includes intracellular and interstitial myocardial edema, capillary leakage, hyperemia, and, in more severe cases, cellular necrosis and subsequent fibrosis.
The ability to characterize myocardial tissue with respect with these pathologic processes has made cardiac MR the primary tool in the noninvasive assessment of patients with suspected myocarditis. Myocardial edema appears as areas of high signal intensity on T2-weighted images. Contrast-enhanced fast spin-echo T1-weighted MR can be used to assess precontrast and early postcontrast gadolinium enhancement (EGE) of the myocardium, which correlates with inflammation, hyperemia, and capillary leak. Myocardial late gadolinium enhancement (LGE) specifically reflects myocardial injury (i.e., necrosis and fibrosis).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree