Fig. 25.1
Importance of the fibrous cap thickness as predictor of the embolic risk
Endothelial Denudation with Superficial Platelet Aggregation and Fibrin Deposition:
Fissured Plaque:
Once a fibrous cap is ruptured, its inner thrombogenic content is exposed and platelet aggregation is originated. Platelet activation and thrombin formation in combination with the plaque content can produce acute vessel occlusion [41]. This explains the importance for diagnosing plaque rupture at an early stage, which can be accomplished by MRI imaging.
Severe Stenosis >90%:
A severe stenosis refers to abnormal narrowing of the artery lumen, which reduces blood flow. Shear stress on the surface of severely stenosed plaque imposes a significant risk of thrombosis and sudden occlusion [42]. Several radiological imaging methods have the potential to diagnose a severe stenosis such as Doppler ultrasound (DUS), computed tomography angiography (CTA), magnetic resonant angiography (MRA), or Digital subtraction angiography (DSA).
2.2 Minor Criteria
Superficial Calcified Nodule:
The superficial calcified nodule refers to a calcification close to the fibrous cap with the potential to cause cap rupture [40]. Calcified nodules might be diagnosed using US, CT, and MRI images.
Yellow Plaque on Angioscope:
Yellow plaques on angioscope are believed to carry a high lipid burden and are, therefore, prone to rupture [40]. As the color is an angioscopic finding, this information cannot be depicted by radiological imaging methods.
Intra-plaque Hemorrhage:
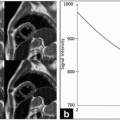
Hemorrhage inside an arteriosclerotic plaque accelerates plaque progression and increases the risk for thromboembolic complications by creating new destabilizing factors such as increase in lipid core and plaque volume [43]. Intra-plaque hemorrhage is considered a minor criterion; however, recent publications cast doubt on this ranking and suggest a more important role [43–45]. Two different types of intra-plaque hemorrhage can be distinguished: type I refers to histologically intact erythrocytes with intracellular methemoglobin and, meanwhile, type II is characterized by lysed erythrocytes and extracellular methemoglobin. MRI can accurately differentiate both types [40].
Endothelial Dysfunctions:
Endothelial impairment plays a major role in the initiation of arteriosclerosis and its progression. Increased adhesion molecule expression and reduced anticoagulant properties increase the risk for cardiovascular disease [46]. Arterial stiffness and endothelial dysfunction commonly coexist and can be assessed by MRI or DUS [46].
Positive Remodeling:
Positive Remodeling refers to an enlargement or growth of an arteriosclerotic plaque without reduction of the luminal area [40]. MRI can assess the positive remodeling.
Radioloical imaging methods should not only be able to detect the degree of a stenosis but must also provide information about superficial structure and morphology of the plaque. Here, the challenge is to identify asymptomatic high-risk patients, differentiate stable from unstable plaques, and predict the risk of a stroke.
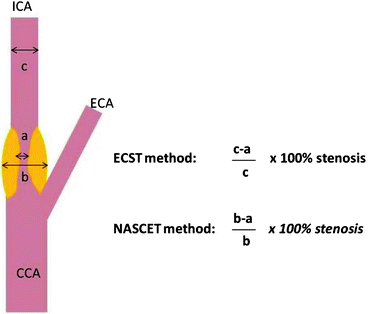

Fig. 25.2
Graphic shows anatomic sites of measurement in the carotid artery for calculating percent stenosis for the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the European Carotid Surgery Trial (ECST)
3 Measurements
The North American Symptomatic Carotid Endarterectomy Trial (NASCET) and European Carotid Surgery Trial (ECST) have established criteria for stenosis quantification of images based on DSA. These criteria are currently used for CTA and MRA to determine vascular diameter and to quantify the degree of carotid artery stenosis. The NASCET method estimates a stenosis by calculating the ratio between the patent lumen diameter at the highest degree of stenosis and the patent lumen diameter at the distal “normal” internal carotid artery beyond the carotid bulb [11]. The ECST method calculates the ratio between the patent lumen diameter at the greatest stenosis and the total outer diameter of the arterial wall at the same level [5]. Comparing NASCET and ECST methods for determining the degree of a stenosis, the NASCET criteria underestimate the degree of a stenosis if compared to ECST; however, the higher the degree of stenosis, the less the difference between both methods [47, 48]. Most of the published trials are based on the NASCET criteria for evaluating the degree of a carotid artery stenosis (Fig. 25.2).
Some limitations have been reported when applying NASCET criteria. Incorrect NASCET measurements on reconstruction images can overestimate the degree of stenosis. It is of crucial importance to correctly choose the distal “normal” ICA for NASCET measurement because the carotid bulb is larger than the outflow ICA. The measurement should be performed perpendicular to the vessel and only in parallel artery walls. Furthermore, for near occlusion NASCET criteria are lacking. The distal ICA diameter is reduced owing to the decreased blood flow in the distal ICA which leads to underestimation of the stenosis degree [11].
4 Indications for Treatment
4.1 General Aspects
Recommendations for the selection of revascularization techniques for patients with carotid artery stenosis mostly depend on the patient’s clinical situation and the presence of comorbid medical conditions and anatomical factors. CEA is an efficient technique to prevent strokes in patients with carotid artery stenosis, and CAS, as a less invasive treatment alternative, has to be compared with the open surgical revascularization procedure. CAS can be performed under local anesthesia; therefore patients with comorbid medical conditions (cardiopulmonary diseases, open heart surgery within 6 weeks, etc.) may benefit from the CAS procedure. CAS has the clear advantage that it does not injure cranial nerves which occurs after CEA [22, 49]. Unfavorable anatomy such as surgically inaccessible high carotid bifurcation has also been considered an indication [50]. CAS should furthermore be performed instead of CEA in carotid artery dissection, tandem lesions, contralateral laryngeal nerve palsy, carotid artery stenosis with occlusion of the contralateral ICA, restenosis after CEA, and stenosis after radiotherapy [50, 51]. CAS should not be performed in patients who have contraindications to dual antiplatelet therapy [50].
4.2 Symptomatic Patient
Patients with carotid artery disease are considered symptomatic when they experience non-disabling ischemic stroke or transient cerebral ischemic symptoms including hemispheric neurological deficit or amaurosis fugax [5]. For these conditions, recommendations have been made for CEA but cannot be transferred to CAS due to lack of scientific evidence. For stenosis >70%, based on the NASCET criteria, operative treatment is strongly recommended; meanwhile symptomatic patients with stenosis >50% will probably benefit from CEA [51]. Other guidelines suggest ICA revascularization if a symptomatic stenosis >70% is documented by noninvasive imaging modalities or >50% as documented by catheter angiography [52]. At present, CAS is recommended in patients who need a revascularization and are at high risk for surgery or as an alternative to CEA, if high-volume centers have a documented death or stroke rate of ≤6% [50]. Both CEA and CAS should be performed in the early period after a symptomatic event, and CEA within 2 weeks thereafter [51, 53].
4.3 Asymptomatic Patient
Meanwhile in the presence of symptoms 5 patients have to undergo surgery to prevent 1 ipsilateral stroke in 5 years; 12 men or 24 women have to be treated if patients are asymptomatic [51]. The selection of asymptomatic patients for carotid revascularization requires an evaluation of comorbid conditions and life expectancy. CEA could be beneficial in asymptomatic patients with >60% carotid stenosis if their life expectancy is >5 years and if the perioperative stroke and death rate for the procedure performed by the surgical team is <3% [50]. In asymptomatic individuals with indication for revascularization, CAS can be an alternative in high-volume centers with a documented stroke and death rate of <3% [50, 51].
4.4 Chronic Total Occlusion
Chronic total occlusion of the ICA usually has a benign and asymptomatic course. Revascularization procedures are currently not recommended because they do not offer any benefit compared with best medical therapy regarding stroke prevention [50, 52]. However, it has been reported that endovascular revascularization can be performed in a very selected group of symptomatic patients despite the risk of distal embolization, vascular rupture, perforation, or hyperperfusion syndrome [52, 54].
4.5 Acute Total Occlusion
On the other hand, revascularization of internal carotid artery occlusion in the setting of an acute stroke is feasible and has shown a high rate of technical success with a favorable clinical outcome (Fig. 25.3). These data indicate that this technique can be useful in patients with severe stroke symptoms if it is performed within the first 6 h [55]. Outcomes after carotid artery stenting in acute stroke depend less on the result of the stent placement than on timing, efficacy, and complications of the cerebral thrombectomy.
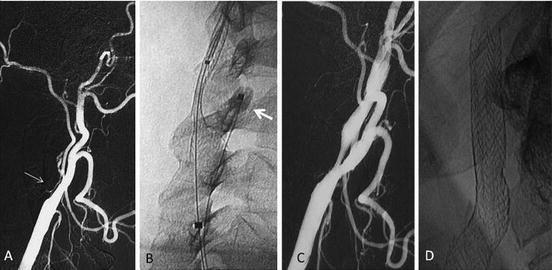

Fig. 25.3
Patient presenting with sudden left hemiplegia. (a) DSA performed within the 3 h shows complete right ICA occlusion (thin arrow). (b) Immediate tentative of revascularization was started using a flow reversal device (Mo.Ma, Invatec) as cerebral protection. See the distal balloon placed in the proximal ECA (arrow); the proximal balloon cannot be seen in this image. (c) Recanalization could be achieved by means of stent placement alone without balloon dilatation in order to avoid a hyperperfusion syndrome. (d) Plain film performed before discharge shows almost complete expansion of stent rendering angioplasty unnecessary
4.6 Near Occlusion
A near occlusion is defined as a carotid artery stenosis of 99% with reduced antegrade blood flow in the ICA and distal lumen collapse (“string sign”). In contrast to complete chronic occlusion, near occlusion burdens the risk of cerebral embolic events. CEA has little or no benefit for reducing future stroke risk compared with medical therapy alone [50, 52]. For CAS, no general recommendation can been made due to lack of scientific evidence; however, CAS in this condition has been reported with a low intraprocedural stroke and death rate and a good stroke prevention at long term [56, 57]. The presence of a sufficient collateral circulation has been discussed in asymptomatic patients, and in this condition, medical treatment is the recommended therapy. However, if recurrent or crescendo symptoms are present despite a medical therapy, an invasive treatment should be considered [58].
4.7 Stenosis Less than 50%
For stenosis <30% CEA increases the 5-year risk of stroke and has no beneficial effect for stenosis ranging from 30 to 49%. Therefore, neither CEA nor CAS is recommended in symptomatic patients with stenosis <50% and optimal medical treatment should be the primary therapy consisting of long-term dual antiplatelet therapy and long-term statins [50]. As said before, vulnerable plaques are likely to cause ischemic events and symptoms can appear even in the presence of low-grade stenosis despite medical management [59] (Fig. 25.3). These patients might benefit from revascularization procedures to prevent further stroke; however, no recommendation can be made and indication for an invasive treatment should be made individually [52, 59].
4.8 Patients Undergoing Cardiac Surgery
Patients with high-grade carotid stenosis undergoing coronary bypass or other cardiac surgeries present with a higher risk of stroke than patients without carotid disease.
The decision to treat an ICA stenosis before cardiac surgery should be discussed by a multidisciplinary team. Optimal time between a carotid intervention and cardiac surgery depends on clinical presentation, level of emergency, and severity of carotid and cardiac disease. With a <6-month history of TIA, revascularization is recommended for stenosis >70%, and can be considered for stenosis 50–69% in certain conditions. For asymptomatic patients, revascularization is only recommended for men who present with a bilateral 70–99% stenosis, an ipsilateral 70–99% stenosis with contralateral ICA occlusion, or previous silent ipsilateral cerebral infarction [50].
5 Noninvasive Imaging
5.1 Ultrasound
Ultrasound (US) is the most important tool for evaluation of carotid artery disease since it is widely available, low cost, and noninvasive. It is operator dependent, and therefore, criteria have been developed for reproducibility and reliability of ultrasound examinations. The carotid arteries should be evaluated using gray-scale, color Doppler, and spectral Doppler examinations.
US Before Stent Placement:
It is of utmost importance to correctly identify the degree of a carotid artery stenosis since indication for a carotid artery intervention depends on the clinical presentation and the degree of a stenosis. Therefore following categories for measuring the degree of ICA stenosis have been suggested: (1) Normal, (2) Stenosis <50%, (3) Stenosis 50–69%, (4) Stenosis ≥70%, (5) Near occlusion, (6) and Total occlusion [60]. For high-grade carotid artery stenosis (stenosis 70–99%) the US examination is an excellent screening method but with less accuracy for low-grade stenosis [61].
The gray-scale US has the potential to localize an arteriosclerotic lesion, measure intima-media thickness, and can identify the morphological degree of a stenosis by differentiating the lumen from the arterial wall. Gray-scale US has furthermore the potential to evaluate the superficial structure of a plaque and to detect plaque ulcer. The echogenicity of a plaque, assessed by gray-scale US, can further determine the risk for ipsilateral ischemic complications, since echolucent plaques are associated with a higher risk of future stroke than echogenic plaques [62]. Due to similar echolucency characteristics, a differentiation between fibrous intra-plaque tissue and the lipid core is not possible and intra-plaque bleeding might not correctly be diagnosed.
The DUS provides functional and hemodynamic information. The DUS can detect flow disturbances in plaque depressions and can be used to calculate the area of stenosis [63]. For area measurements US images should be obtained in a transverse fashion, since arteriosclerotic plaques can be eccentric. For determining the degree of a stenosis, the spectral Doppler examination is essential. The primary criterion for diagnosing an ICA stenosis is the ICA peak systolic velocity (PSV) in combination with the gray-scale evaluation [60]. If the stenosis is uncertain for the primary parameters, then additional parameters are used, such as ICA end diastolic velocity (EDV) and the ratio of ICA PSV/CCA PSV [60] (Table 25.1). The evaluation of ICA/CCA PSV is especially important in certain conditions such as aortic stenosis, low cardiac output, or detection of contralateral carotid occlusion [64].
Degree % | ICA PSV (cm/seg) | Plaque estimate % | ICA/CCA PSV (cm/seg) | ICA EDV (cm/seg) |
|---|---|---|---|---|
Normal | <125 | None | <2.0 | <40 |
<50 | <125 | <50 | <2.0 | <40 |
50–69 | 125–230 | ≥50 | 2.0–4.0 | 40–100 |
≥70 | >230 | ≥50 | >4.0 | >100 |
Near occlusion | High, low, indetectable | Visible | Variable | Variable |
Total occlusion | Undetectable | Visible, no detectable lumen | Not applicable | Not applicable |
For correct evaluation of flow velocities, technical aspects have to be considered. The PSV should be measured after a regular heartbeat. For determining flow velocities, a transversal view is preferable and the pulsed Doppler sample volume should be placed precisely within the area of the maximum stenosis. A wrong angulation of the Doppler gate leads to erroneous flow velocities. Therefore it has been recommended to use an angle within 45–60%, which ensures minimizing errors of flow velocity measurements [65]. In tortuous vessels the cursor should be placed tangential to the curvature of the artery.
US After Stent Placement:
The US is the standard technique for screening after stent placement, to detect a residual stenosis or restenosis due to hyperplasia. Gray-scale US may provide morphologic information about the degree of a stenosis; however, blood flow velocities for stenosis detection, as previously described, fail. A “normal” carotid artery has an elastic vessel wall, which is reflected in the low-resistance waveform of a spectral DUS. After stent placement the artery will lose this condition due to the rigid device. Furthermore, wall stress produced by the stent can cause endothelial dysfunction, which is responsible for hyperplasia and restenosis. For this reason, the above-mentioned blood flow velocities for the evaluation of in-stent restenosis are not applicable. Although different thresholds for blood flow velocity have been proposed, it could have been demonstrated that current velocity criteria to assess in-stent restenosis after CAS yield a high false-positive rate [66]. Baseline control within 48 h after stent placement should be performed with consecutive control to document changes in blood flow velocities over time rather than applying absolute velocity criteria [67, 68]. If an in-stent restenosis is suspected, other imaging modality such as CTA has been proposed to confirm the degree of stenosis [69].
US Limitations:
US can be of limited value in special conditions. Severe calcifications produce ultrasonic shadows, which limits the morphologic evaluation of the gray-scale US. For the same reason, the DUS might not be able to assess blood flow velocity at the greatest stenosis. In this condition, velocity measurements proximal and distal to the shadow could determine the degree of the stenosis.
Anatomic patient conditions influence quality of the US image. A high carotid artery bifurcation might not be assessed as well as in obese patients with a short neck. Tandem lesions might not correctly be depicted on gray-scale US evaluation; however, if there is inconsistency between a severe stenosis diagnosed on gray-scale US and the distal spectral waveform detected by DUS, an upstream lesion has to be suspected. Anatomic variants might cause confusion such as carotid artery kinking. In this condition it might be difficult to distinguish the external from the internal carotid artery. Elevated flow velocity and high-resistance flow pattern as well as outgoing branches help to differentiate the external from the internal carotid artery. US has a limited value for discriminating near occlusions from total occlusions [70]. The lack of detection of a residual flow might lead to false-positive results. Alternative imaging modalities such as DSA, MRA, or CTA can be useful to differentiate near from total occlusions.
5.2 Computed Tomography
The CT is a noninvasive technique for the workup of carotid artery stenosis and for follow-up after carotid stent placement. Meanwhile a non-enhanced CT has the potential to detect ischemic and hemorrhagic cerebral complications; the contrast-enhanced CTA can be used to determine the degree of a carotid artery stenosis and to evaluate the intracranial circulation. Currently, the mutidetector CT angiography (MDCTA) permits a fast evaluation with a high spatial resolution. Different reconstruction algorithms such as three-dimensional (3D) reconstructions, major intensity projections (MIP), and multiplanar or curved reconstruction images have contributed to improved accuracy [71].
CT Before Stent Placement:
CTA is a valuable method for carotid artery disease evaluation [72]. Since MDCTA was used to diagnose ICA stenosis, improved accuracy has been demonstrated [73]. The highest accuracy was reported for detecting complete ICA occlusion [74]. MDCTA can furthermore discriminate total from near occlusion, with an excellent correlation with catheter angiography [75]. For high-grade stenosis (70–99%) without near occlusion, the CTA is an accurate method [74]. In contrast to high-grade stenosis, the diameter of the distal ICA is reduced in near occlusion, which can accurately be depicted by MDCTA. This is of special importance since patients with severe stenosis and a normal distal ICA but no near occlusion are at higher risk to develop a future stroke. Quantitative millimeter measurement of ICA stenosis has also been proposed to estimate the risk of stroke and to overcome ambiguities with NASCET criteria [76, 77]. Special care has to be taken in the group of 50–69% stenosis because symptomatic patients would receive invasive treatment and meanwhile patients with stenosis <50% receive medical treatment only. Here, CTA has the lowest specificity, compared to other noninvasive imaging methods [78]. On the other hand, CTA has an excellent negative predictive value for demonstrating <70% stenosis [79].
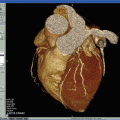
CTA can provide morphological information about the vascular wall and atherosclerotic plaques. It is very sensitive for detection and measurement of carotid plaques calcification and plaque ulcerations [80, 81]. Different plaque components such as fibrous connective tissue and fat may be distinguished from plaques containing calcifications due to their dissimilar densities (Fig. 25.4).
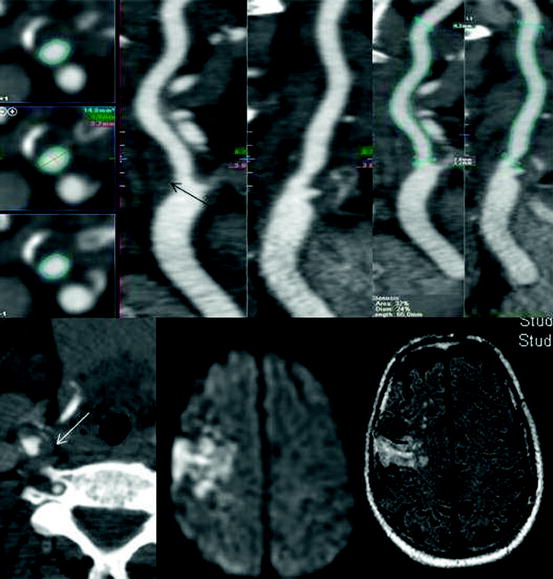

Fig. 25.4
Soft plaque depicted by CT (arrows). Although the stenosis measures by NASCET were not significant (<50%), this plaque embolized causing a right hemisphere infarct
However, this differentiation is not possible for the lipid core, fibrous tissue, and intra-plaque hemorrhage. The Dual-Energy-CT is a new CT technique that uses two independent radiation sources with different voltages and has the potential to identify patients with vulnerable plaques better than conventional CTA [82–113]. Dual-Energy-CT is therefore a valuable tool for the noninvasive assessment of different plaque components and can discriminate mixed plaques from low-density fatty plaques [83].
CT After Stent Placement:
The MDCTA has emerged as a noninvasive modality for surveillance after stent placement [84, 85]. DUS is the primary choice for assessing in-stent restenosis. However, it is of limited use because not all stents are assessable due to severe calcifications with dorsal sonic shadowing or due to anatomic localization. Here the CTA has proven to be an alternative for in-stent restenosis detection. Intimal hyperplasia on CTA images appears as variable hypodense ring between the hyperdense stent and the contrast filled artery lumen. Compared to DUS, MDCTA shows comparable results regarding detection of in-stent restenosis [68]. However, the dense metal struts can lead to local artifact that can degrade the image in the immediate vicinity of the vessel wall, which leads to overestimation of the degree of stenosis [50]. Other limitations of CTA imaging include motion artifacts, small stent diameter surrounded by heavy calcifications, and strike artifacts caused by the stent or stent markers such as tantalum or gold [86]. This is of extreme importance since artifacts caused by stent markers can masquerade as in-stent restenosis which is frequently found at the extremities of a stent. Therefore, the MDCTA has been recommended as an alternative technique if the stent is not completely assessable by DUS.
CT Limitations:
The CTA has certain limitations. Even if it is a noninvasive method, iodine contrast has to be applied, which burdens the risk of contrast-induced nephropathy and the potential for allergic contrast reactions. Furthermore, the patient is exposed to ionizing radiation. Image quality might be limited in the uncooperative patient due to motion artifacts or in case of severe obesity. Metal structures such as surgical clips, endodontic prosthesis, or even vascular stents cause strike artifacts that may impede an appropriate evaluation of the patent vascular lumen. Very hyperdense structures like heavily calcified lesions, especially if circumferential, cause a hardening effect, which results in overestimation of the hyperdense structure and underestimates the lumen diameter [87].
5.3 Magnetic Resonance Imaging
With the technical advances of the past years the MRI has become a valuable noninvasive screening tool for detection of carotid atherosclerotic disease. Meanwhile the CT is the preferred method to differentiate a hemorrhagic from an ischemic stroke; the MRI is superior for early detection of ischemic lesions [88]. MRI has also an excellent soft-tissue contrast, which makes it a valuable technique for evaluating the vulnerability of a carotid plaque [40, 83]. MRI has further been used for the follow-up after carotid stent placement [84].
MRI Before Stent Placement:
Some technical aspects have to be considered when using this technique. A 1.5 T MRI might be sufficient for an adequate vascular screening of the extra- and intracranial structures; however 3 T MRI machines provide better spatial resolution. For the assessment of plaque morphology, phased-array superficial coils must be used to optimize signal-to-noise ratio [85].
The MRI provides cross-sectional images in any plane including oblique planes. For detection of the degree of a stenosis, different sequences have been applied such as contrast-enhanced MR angiography (CE-MRA), time-of-flight (TOF) MRA, and phase-contrast MRA [89]. Since CE-MRA is fast, it has become the technique of choice for studying the carotid bifurcation [90]. The flow-dependent TOF MRA or phase-contrast MRA is of less importance due to their distinct susceptibility to motion or flow-related artifacts [91]. On the other hand, TOF MRA is the recommended technique for evaluation of steno-occlusive disease in the intracranial arteries, since it has a higher spatial resolution compared with CE-MRA [92, 93].
For the grading of a carotid artery stenosis CE-MRA is an excellent technique. For detecting carotid artery disease, CE-MRA is comparable with CTA and DUS. For detecting severe stenosis (70–99%) it is superior to CTA and DUS and is the most accurate method [78, 94–96]. CE-MRA is also very accurate for detecting total occlusions and can precisely discriminate near from total occlusion [70, 97]. For moderate ICA stenosis CE-MRA and especially TOF MRA appear to be poor diagnostic tools [97].
MRI is the preferred technique for the evaluation of stroke or transient ischemic attacks before and after stent placement, because it is the most sensitive technique for detecting early ischemic complications [88]. Diffusion-weighted images (DWI) should be obtained since patients who present with lesions visualized in DWI, especially when multiple, are at higher risk of recurrent ischemic events [88].
In the last years different MRI protocols have been evaluated for detecting different plaque characteristics. A multi-sequence MRI protocol has been proposed by different authors and can be used as a guide to assess the vulnerability of a carotid plaque [37, 98]. T1-weighted images before and after the application of contrast, proton density (PD)-, and T2-weighted images as well as TOF help to differentiate morphological characteristics [37]. Fat suppression should be used in all sequences to avoid signal from the subcutaneous fat.
For identifying an active inflammation of a carotid plaque, contrast has to be used and the inflammatory process can be detected by contrast enhancement of the vascular wall [40]. The lipid-necrotic core can accurately be differentiated from the surrounding tissue comparing pre- and post-contrast T1-w images since the lipid-necrotic core does not show enhancement after contrast [99]. The fibrous cap is hyperintense relative to the lipid-necrotic core after contrast on T1-w images [100, 101]. Calcified nodules appear hypointense on all 4 weightings and can be differentiated from the vascular lumen after the application of contrast or might be distinguished from the bright lumen in the TOF images [100, 102]. Intra-plaque hemorrhage can appear hyperintense on TOF and T1-w images and iso- or hypointense on PD- and T2-weighted image if type I hemorrhage is present but can have a high signal intensity on all sequences in case of type II hemorrhage [100].
MRI After Stent Placement:
CE-MRA has been reported for the follow-up as it has the potential to visualize the patent stent lumen [103, 104]. Two techniques have been used to evaluate carotid stents, the CE-MRA and TOF MRA. The main problems of MRI are stent-related artifacts that lead to artificial lumen narrowing [84]. Artifacts depend on the stent material; here, different magnetic susceptibility characteristics of the stent influence the image quality [84, 105]. On the other hand, stent geometry and size of stents have an impact on the visualization of the stent lumen [84]. Currents induced inside the stent struts reduce signal strength leading to artificial lumen narrowing. In contrast to CE-MRA, the TOF MRA is based on rapid radiofrequency pulses, which furthermore increases susceptibility artifact [104]. For this reason, MRA in the follow-up after carotid stent placement has only limited value.
MRI Limitations:
MRI is not as widely available as CT and is generally more expensive. It is contraindicated in patients with pacemakers, cochlear implants, neurostimulators, and metallic foreign objects. The application of contrast carries the risk of allergic reactions and renal complications, including nephrogenic systemic fibrosis [84]. Claustrophobic patients might need sedation due to the long and narrow gantry. To entirely assess extra- and intracranial vascular structures and plaque morphology and evaluate intracerebral lesions, it is a time-consuming method. Acquisition time of certain MRI sequences might be long and MRI is therefore sensible to motion artifacts. As described previously and depending on the sequences used, image quality can be restricted by factors, such as limited spatial resolution or scan range, flow signal intensity loss as a result of saturation or phase dispersion, and susceptibility artifacts [106]. Furthermore, MRA is reported to overestimate the degree of ICA stenosis, especially in non-contrast sequences such as TOF [50, 71].
Until recently, indication for carotid revascularization was related to patient symptoms and the degree of stenosis assessed by imaging techniques measuring the vessel’s caliber, giving no information on plaque characteristics (stable or instable “vulnerable plaque”).
Recent studies demonstrate how a 40% stenosis with a 0.2 mm thick “fibrous cap” is associated with the same wall stress and has risk of rupture as an 80% stenosis with a 0.5 mm thick “fibrous cap” [107].
In the next years, treating patients with “high-risk plaque morphology” will become a proven practice and standard of care supported by improvements in CT and MRI characterization of plaques.
6 Techniques of Carotid Artery Stenting
The efficacy of CAS in preventing stroke depends on careful patient selection and technical details. Although CAS is generally associated with low intraprocedural and post-procedural adverse neurologic events, it is mandatory to continuously undertake efforts to further reduce the rate of adverse events because these have a major effect on patient outcome.
Techniques and materials used for CAS are constantly evolving in order to make this procedure effective and safer.
One major concern with CAS is the potential distal embolization of particles from the treated site during the procedure. Embolic particles are classified as either macroemboli (>150 μm) or microemboli (<150 μm). Macroemboli, especially >200 μm, are associated with neurological events. To avoid this, various cerebral embolic protection devices (EPDs) have been developed and improved during the last years. However, published data to date do not clearly demonstrate the usefulness or safety with respect to clinical complications [108]. The use of EPDs, for some authors including us, increases the procedural complexity and risk of embolization during device manipulation. Since EPDs are expensive and prolong procedure time, it is important to determine the clinical value of this practice [109].
In 2000 our group started to treat carotid stenoses treated by CAS without using EPDs and without performing balloon dilatation neither before nor after stent placement, with encouraging results in the short and long term [110–112].
Herein, we will provide a step-by-step approach to the clinical and technical aspects that ensures the safe performance of carotid stenting. This includes the technique mostly performed worldwide with the use of EDPs and balloon angioplasty; the less used technique in which balloon angioplasty and stenting are performed without neuroprotection; and finally we will describe the author’s technique for CAS without neuroprotection and balloon angioplasty, and discuss, from our point of view, the advantages of it.
6.1 Considerations for Safe CAS
6.1.1 Clinical Protocol (Table 25.2)
Table 25.2
Clinical protocol
Clinical protocol |
|---|
Pre-procedure: |
• Carotid Duplex Ultrasound |
• 4-Vessel DSA (whenever possible previous to procedure) |
• MRI with DWI. If not possible CT-Scan |
• Neurological evaluation |
• ASA 100 mg PO bid × at least 3 days |
• Clopidogrel 75 mg or Ticlopidine 250 mg PO bid × at least 3 days |
Procedure: |
• No sedation |
• 5,000 IU of heparin IV bolus |
• Squeezing toy in contralateral hand |
• Placement of devices directly in the carotid to be treated |
• Sheath removal |
Post-procedure: |
• Post-procedural neurological evaluation |
• Carotid Duplex Ultrasound |
• MRI with DWI. If not possible CT-Scan within 48 h post-procedure |
• ASA 100 mg PO bid × life |
• Clopidogrel 75 mg × 3 months |
• Clinical and US F/U: 1, 3, 6, 12 month, annually thereafter |
All patients should undergo a neurologic examination by a neurologist in order to document the pre-procedural clinical status using the baseline N.H.I.S.S (National Institute of Health Stroke Scale) or other functional scales.
The indication for stenting is given by DUS and DSA in most of the cases. In selected cases CTA or MRA can be also performed.
We pay close attention to the morphological aspect of the plaque which is assessed by ultrasonography. The plaque morphology is classified into 4 grades: grades 1 and 2 (echolucent or predominantly echolucent plaque) are considered as high-risk plaque morphology; grade 3 (predominantly echogenic) is medium risk, and grade 4 (echogenic) was low risk for stroke [5]. In our protocol, all patients with a high-risk plaque are treated if stenosis was more than 50%. Consider, if available, plaque characterization with MRI or CT (see high-risk plaque).
Angiography is performed prior to the endovascular intervention. The degree of stenosis before stenting is quantified using the NASCET criteria [6].
Before treatment, and whenever possible, a baseline cerebral MRI including diffusion weighted images (DWI) is recommended. If contraindicated or not available, CT should be done.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree