Carpal Fractures and Dislocations
Carpal Instability
Carpal instability, literally defined, occurs when the wrist demonstrates symptomatic malalignment, is unable to bear loads, and has abnormal kinematics during any portion of its arc of motion. This can occur as an acute condition following injury or as a chronic, ongoing process as seen in patients with inherent excessive ligamentous laxity or ligament attenuation from inflammatory arthropathy. Because this book addresses traumatic conditions, this chapter focuses on carpal instability as a result of acute injury to osseous and key ligamentous components of the wrist. Such conditions are often referred to as “perilunate injuries.”
Prior to any discussion of carpal instability, a brief review of the key anatomic components of the wrist required for stable, coordinated motion is in order. After all, surgical management of these conditions is aimed at restoring the functional integrity of these structures. For simplicity, the carpal bones are traditionally organized into proximal (scaphoid, lunate, triquetrum, and pisiform) and distal (trapezium, trapezoid, capitate, and hamate) rows. The static and dynamic relationship between these bones is maintained by those ligaments within (intrinsic) and those spanning (extrinsic) each row. The proximal carpal row is an intercalated segment void of any tendon insertions, rendering the motion and position of the scaphoid, lunate, and triquetrum entirely dependent on surrounding articulations and ligament integrity.
Extrinsic ligaments arise from the dorsal and volar aspects of the distal ulna and radius to insert on the proximal and distal carpal rows. The stout palmar ligaments (Fig. 20.1), from radial to ulnar, consist of the radioscaphocapitate (RSC), the long radiolunate (LRL), the short radiolunate (SRL), and the ulnocarpal ligaments. The RSC spans the volar waist of the scaphoid before inserting on the capitate and acts as a fulcrum for the scaphoid as it flexes and extends with radioulnar deviation. It and the adjacent LRL, which joins the volar rim of the radius and the volar lip of the lunate, suspend the radial side of the carpus and prevent it from “sagging” or palmarly subluxing relative to the radius. The SRL extends from the volar rim of the lunate fossa to the lunate, inserting just ulnar to the LRL. The ulnocarpal ligaments originate from the volar periphery of the triangular fibrocartilage complex to insert on the lunate and triquetrum. They function to support the ulnar carpus and prevent it from sagging palmarly or subluxing relative to the distal ulna.

On the dorsum of the wrist, the dorsal radiocarpal (DRC) ligament spans from the central dorsal distal radius with the joint capsule to attach to the dorsal aspect of the triquetrum. The dorsal intercarpal (DIC) ligament connects the dorsal triquetrum to the dorsal surfaces of the scaphoid and trapezium (Fig. 20.2).
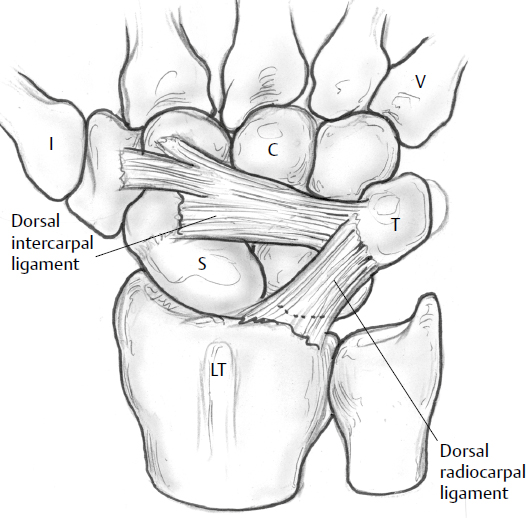
The intrinsic ligaments link the scaphoid, lunate, and triquetrum to enable flexion and extension of the proximal carpal row simultaneously with wrist radial and ulnar deviation (Fig. 20.3). The scapholunate ligament is noted to have three morphologically distinct regions, the dorsal aspect being the thickest.1,2 Conversely, the lunotriquetral ligament is more stout on the palmar side.3

Various theories have been proposed to explain the kinematics of wrist motion, and there is no need to cover the full details in this chapter. The key is to understand that the proximal and distal carpal rows rely on a contiguous, stable ligament and osseous link among and between them to provide reciprocating motion relative to each other (Fig. 20.4). This is often referred to as the “ring” concept.4 With ulnar deviation, the proximal row extends as the distal row flexes. The opposite occurs with ulnar deviation. Structural failure of any bone or ligament portion of this linkage within the proximal row results in loss of coordinated motion of the carpus with the possibility of progressive collapse, painful arthrosis, and disability. The goal of surgical management is restoration of this linkage.

Classification
Not all injuries resulting in carpal instability are readily apparent on plain X-rays. Static instability refers to those injuries visible in standard radiographs. This may take the form of a carpal fracture, widening of scapholunate or lunotriquetral joints, or excessive scapholunate angle on the lateral view. Dynamic instability requires stress maneuvers such as clenched fist or ulnar deviation to demonstrate pathology on plain films or fluoroscopy.1
Perhaps the most widely shared classification of perilunate injury that links injury mechanism and sequential failure of anatomic components is that described by Mayfield and colleagues.5 When the wrist is loaded during a fall, it is often in a position of extension and ulnar deviation, resulting in progressive supination of the carpus relative to the radius. As the loaded wrist continues to supinate, osseous or ligamentous failure begins with the radial aspect, proceeds through the carpus, and exits the ulnar side of the wrist. Therefore, Mayfield proposed four stages of progressive perilunar instability as follows:
Stage I: Rupture of scapholunate and palmar radioscaphocapitate ligaments. This would appear as widening of the scapholunate interval (> 3 mm) on normal or stress views of the wrist, with perhaps an increased scapholunate angle (> 60 degrees) on the lateral X-ray (Fig. 20.5).
Stage II: Dislocation of the capitolunate joint.
Stage III: Disruption of the lunotriquetral and ulnocarpal ligaments, as seen in a perilunate dislocation, in which the lunate remains in the lunate fossa of the radius while the remainder of the carpus displaces dorsally (Fig. 20.6).


Stage IV: Lunate dislocation, during which the dorsal radiocarpal ligament and capsule fail to allow lunate displacement palmarly into the carpal canal. It is usually still tethered palmarly by the stout radiolunate ligaments, resulting in the “spilled teacup” appearance on the lateral X-ray (Fig. 20.7). The remainder of the carpus falls back into a collinear position with the radius, as opposed to being dorsal to it in perilunate dislocations seen in stage III.
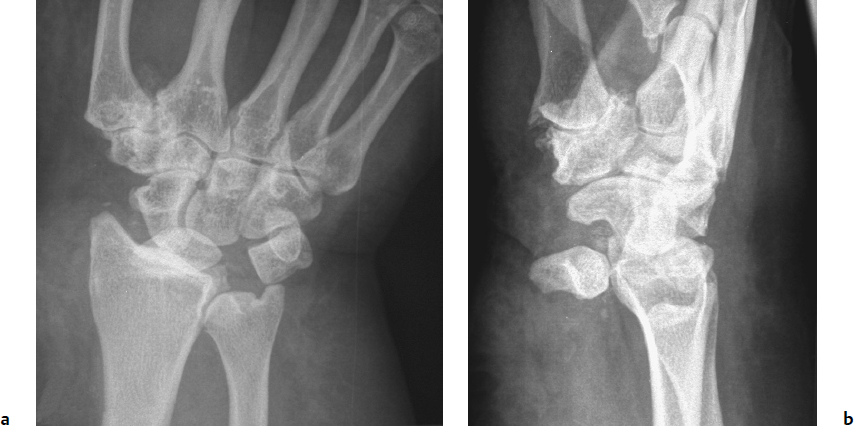
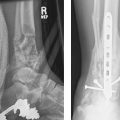
Note that the foregoing classification only addresses ligamentous disruption, which does not always occur in isolation as the force passes through the carpus. Beginning with the radial side, fractures of the radial styloid, scaphoid, capitate, triquetrum, and ulnar styloid may occur with force transmission (Fig. 20.8). Greater arc injuries are fracture patterns in which the force takes a wider, transosseous path through the carpus. Lesser arc injuries are those that involve a narrower pathway of soft tissue ligamentous failure around the lunate, as described by Mayfield. Inferior arc injuries result from forces passing proximal to the lunate, resulting in disruption of radiocarpal and ulnocarpal ligaments with or without radial or ulnar styloid fractures. Acute carpal instability frequently involves both bony and ligamentous components, such as those seen in the stage III transscaphoid-perilunate dislocation (Fig. 20.9). These injuries frequently result in fractures of the carpal bones themselves as the force passes over and around the lunate.


Isolated lunotriquetral instability, also referred to as a reverse perilunate injury, is thought to occur as a result of a fall on the radially deviated outstretched hand (recall that the hand is typically in ulnar deviation during perilunate injuries). Failure of the dorsal and central portions of the lunotriquetral ligament is insufficient to result in altered carpal mechanics. However, failure of the palmar portion of the ligament, combined with loss of secondary dorsal capsular (radiotriquetral and scaphotriquetral) ligaments, results in progressive static volar intercalated segmental instability (VISI) deformity (see later discussion).
The proximal carpal row, without the scaphoid acting as a stable link or strut bridging against compressive forces across the wrist, would simply collapse into flexion or extension as the distal row migrated proximally. A dorsal intercalated segmental instability (DISI) deformity occurs when the lunate assumes an extended position on the lateral radiograph relative to the radius as the capitate migrates proximally. Likewise, a VISI deformity occurs when the lunate appears flexed relative to the radius as the capitate moves proximally.
In the setting of posttraumatic wrist pain with or without visible clinical deformity, understanding of the carpal relationships on standard posteroanterior (PA) and lateral radiographs will prevent missing a perilunate injury. Any disruption or gapping in the three arcs of Gilula on the PA radiograph should raise suspicion for ligament or osseous injury (Fig. 20.10). The referenced article by Gilula6 offers an excellent approach to carpal injury radiographs.

Scapholunate widening > 2 mm on PA view, > 60 degrees scapholunate angle on lateral view, or loss of a collinear relationship between the radius, lunate, and capitate are hallmark features of perilunar instability. Ulnarly deviated PA or oblique carpal views may also demonstrate scaphoid fractures not seen on routine orthogonal views.
Nonoperative Treatment
Load transmission across the carpus traverses the two mobile carpal rows that must be able to maintain a stable relationship in any posture. This relies on the integrity of the scaphoid, acting as a rigid strut or link between the two rows, and the intrinsic ligaments joining the scaphoid, lunate, and triquetrum. Failure of any of these components results in progressive carpal collapse from baseline compressive forces of the musculotendinous units crossing the wrist and the bony anatomy of the distal radius and carpus itself. Vigorous external loads are not required to cause progressive collapse of the proximal row with subsequent proximal migration of the distal row and secondary arthritic changes. Therefore, there is little basis for the non-operative treatment of these injuries. With the exception of a truly nondisplaced, stable scaphoid waist fracture or partial ligament tear, these injuries simply do not heal reliably with immobilization alone.7,8
Although surgical stabilization is the definitive treatment for these injuries, a provisional reduction in the acute ambulatory setting is often necessary. In both perilunate and lunate dislocations, there may be considerable pressure on the median nerve from the lunate. Patients often have median nerve sensory deficits, and allowing the wrist to remain in an unreduced position with ongoing pressure on the nerve may have deleterious effects. A closed reduction with minimal anesthesia can usually be performed at the initial presentation in a manner similar to that of dorsally displaced distal radius fractures.
With or without adjunctive intravenous sedation, traction is applied through the digits either with the use of finger traps or manually with countertraction on the arm proximally. While applying volar pressure on the lunate with a thumb, the capitate is initially extended and then translated palmarly to engage the distal articular surface of the lunate (Fig. 20.11). With reduction, an audible or palpable “clunk” might not occur. Fluoroscopy or plain X-rays are needed to verify the reduction. A sugar-tong splint molded to keep the wrist from extending should be applied until the time of definitive surgical treatment.

Occasionally, closed reduction of a perilunate or lunate dislocation is not possible. In ligamentous injuries in which the lunate alone is separated from the remainder of the carpal bones, the lunate may buttonhole through the stout volar capsule (Fig. 20.12). With traction applied during the reduction maneuver, the capsular rent merely tightens further to prevent the lunate from returning to its anatomic position relative to the carpus. In these situations, open reduction must be performed. In addition, some transosseous perilunate injuries are so unstable that the reduction cannot be maintained following the release of traction, despite the application of a well-molded splint. Although surgery remains the definitive, necessary treatment to restore stability in these injuries, failure of closed reduction does not always mandate immediate operative treatment. If swelling is minimal and there are mild or no subjective sensory deficits in a closed injury, it is permissible to leave the irreducible dislocation in that position until a more convenient scheduling time. However, should the patient develop progressive sensory loss with marked swelling, nerve decompression through a carpal tunnel release and open reduction becomes more emergent.

Surgical Treatment
Video 20.1 Repair of Perilunate Dislocation
Indications
As already emphasized, all of these injuries are quite unstable, rendering closed treatment and external immobilization rather ineffective. Although a closed reduction may be achieved through manipulation and traction, carpal collapse and proximal migration tend to occur in both transosseous and transligamentous varieties. In general, operative treatment is best performed within 2 weeks of injury. Beyond this interval, capsular scarring ensues and requires significant bone and soft tissue stripping to mobilize the bones adequately to obtain reduction.
Also, as suggested in the preceding section, emergent carpal tunnel release should be performed in the setting of significant swelling with progressive numbness or motor deficit.
Surgical Anatomy and Options for Treatment
Preoperative planning begins by ascertaining the nature and full extent of the injury. Recall that carpal instability represents a spectrum of structural failure as the deforming load passes through the wrist. All components of injury may not be apparent on initial plain films. For example, a small radial styloid fracture noted on initial films may be the only suggestion of the more problematic and significant scapholunate tear or scaphoid fracture. The need for meticulous physical examination (tenderness over scapholunate or lunotriquetral ligaments) and perhaps further radiographic stress views cannot be overemphasized. Traction films taken at the time of initial assessment can be quite helpful (Fig. 20.9).
Surviving the Night
The only reasons to intervene emergently are acute carpal tunnel syndrome (CTS) and, of course, open injuries. Acute CTS has much more dramatic presentation than that seen with both typical chronic, idiopathic CTS and median nerve contusion, a common feature of perilunate injuries. The patient with acute CTS demonstrates rapidly progressive, painful, unrelenting dysesthesias in the median nerve distribution with corresponding sensory and possible motor deficits due to acute nerve ischemia. This should be treated as a surgical emergency, similar to compartment syndrome, and requires emergent carpal tunnel release through a volar approach. Although definitive carpal stabilization is not necessary at this time, reduction of the lunate or other carpal fragments that may have buttonholed through the volar capsule should be performed. Patients with median nerve contusion will have sensory deficits without the dramatic, painful median dysesthesias seen in acute CTS.
A strategy for exposure and repair can be derived following assessment of the injured components. Larger radial styloid fractures should be repaired to restore articular congruity as well as the suspensory role of the radioscaphocapitate ligament. Fractures of the scaphoid or disruptions of the scapholunate ligament require open reduction and rigid fixation or direct ligamentous repair. Capitate or triquetrum fractures also require open reduction and rigid fixation. There is some debate regarding the need for direct repair of the lunotriquetral ligament. Many surgeons feel that restoration of the anatomic relationship of the lunotriquetral joint with Kirschner wire (K-wire) fixation alone is sufficient without performing direct repair of the ligament through bone tunnels or anchors. Others, however, recommend using a combined dorsal and volar approach to enable direct repair of the more significant palmar portion of the lunotriquetral ligament. An ulnar styloid fracture that is large enough to involve the entire insertion of the deep portion of the triangular fibrocartilage should be repaired. Nevertheless, the presence of an ulnar styloid fracture in the setting of a bony or ligamentous radial-sided wrist injury should alert the examiner to the likelihood of more subtle injuries within the carpus.
If the patient manifests any features of median nerve contusion, I prefer to perform a carpal tunnel release at the time of carpal stabilization, even if the reduction could be achieved solely through a dorsal approach.
Surgical Techniques
Proper room setup and equipment preparation are essential. The patient is placed in the supine position on a standard table with a stable arm board attached. Either standard or mini–C-arm fluoroscopy with accompanying sterile draping will be needed, but the arm board does not have to be radiolucent. Many surgeons prefer to tilt the mini–C-arm parallel to the floor, avoiding the need to image through the table. K-wires (0.045- and 0.062-inch) and a drill with an appropriate size attachment will be needed. A chuck and accompanying chuck attachment for the drill will be needed for placement of compression screws. Although virtually any headless compression screw system will suffice, we often use the mini–Acutrak 2 (Acumed LLC, Hillsboro, OR) screw. Whichever screw system is used, the screw needs to be cannulated to allow accurate placement of a guide pin prior to insertion. If ligament repair is to be performed, we prefer to use small (2- to 4-mm) suture anchors. Finally, a 14-gauge metal cannula or catheter (Fig. 20.13) will facilitate accurate placement of percutaneous wires while minimizing the risk of entangling surrounding soft tissues (a painful superficial radial nerve or dorsal sensory ulnar nerve neuroma can undermine an otherwise successful skeletal result). Use of a tourniquet is most helpful but not mandatory. Maintaining reduction while passing fixation wires or screws usually requires all four hands of the surgeon and assistant, making it difficult to repeatedly stop and apply suction to the wound for visualization. Most of these procedures can be performed within 2 hours of tourniquet time.

With the exception of larger radial styloid or ulnar fractures, a single dorsal incision is usually sufficient. A palmar incision will also be needed in the setting of an acute carpal tunnel syndrome or dislocated lunate that cannot be reduced through capsular release via the dorsal approach.
Whether a longitudinal or transverse dorsal incision is used is a matter of personal preference. The transverse incision provides exposure of the entire carpus along with the radial and ulnar styloids. The transverse scar is also less noticeable than a longitudinal one. However, access to the radial or ulnar metaphysis, should it become necessary, is limited. Our preference is a longitudinal dorsal incision passing directly over Lister′s tubercle. Exposure for fixation of radial and ulnar styloids is accomplished through separate longitudinal incisions over each with little risk for flap necrosis (Fig. 20.14).

For the longitudinal dorsal incision, incise the skin 4 to 6 cm in line with Lister′s tubercle centered over the proximal carpal row, creating full-thickness flaps down to the level of the extensor retinaculum. Incise the distal portion of the retinaculum in line with the extensor pollicis tendon. Releasing a portion of the septum between the third and fourth compartments facilitates broader exposure of the dorsal capsule with a self-retaining retractor, reflecting the second- and third-compartment tendons radially and the fourth compartment ulnarly. If an existing post-traumatic tear is present in the dorsal capsule, try to incorporate it into the dorsal capsulotomy. Otherwise, make a full-thickness incision in the capsule in line with Lister′s tubercle for 2 to 3 cm. If the scapholunate ligament is intact, overzealous penetration with the blade during this incision may result in an iatrogenic division of the ligament. Therefore, once through the capsule, lay the blade flat to elevate it from its dorsal attachments on the carpal bones. Avoid excessive dorsal stripping distally on the scaphoid, because this may interrupt its main vascular supply along the dorsal ridge. This incision should provide adequate exposure of the dorsal carpus from the dorsal pole of the scaphoid to the radial margin of the triquetrum. Applicable reduction and stabilization techniques will depend, of course, on the degree and components of the individual injury.
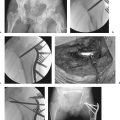
Transscaphoid injury patterns traverse either the waist or the proximal pole of the bone. Placement of temporary 0.045-inch K-wires dorsally in both fragments to serve as joysticks for fragment manipulation and reduction is beneficial (Fig. 20.15). Gently debride the hematoma from the fracture site with a dental pick and irrigation. While one surgeon obtains and maintains the reduction by using the wire joysticks, the other passes a guidewire from the cannulated compression screw set across the fracture site down the central third of the scaphoid (Fig. 20.16). Correct position of this pin is critical. The starting point is generally at the dorsal ulnar “corner” of the scaphoid. Use the sterile-draped C-arm to help with correct placement of the pin down the central third axis, stopping intermittently to verify position on PA, oblique, and lateral views. When checking the PA view, avoid the tendency to extend the wrist to the point where the dorsal rim of the radius impinges on and bends the wire. The distal tip of the wire should not enter the distal cortex of the scaphoid. Once satisfactory depth and position of the guide pin are verified, measure the depth of the pin with either the accompanying depth gauge from the set or an equal-length pin in the set. The selected screw size needs to be ~ 4 mm less than the measured depth of the guide pin. Selecting a screw size that is too long is a common error.


Once the screw length has been determined, pass the guide pin across the scaphotrapezial joint, seating it in the trapezium to avoid inadvertent withdrawal of the wire when using the cannulated drill. To avoid rotational displacement that tends to occur with drill and subsequent screw passage, pass a supplemental 0.045-inch K-wire across the fracture in a line that will not obstruct the other instruments. Using the cannulated hand drill, drill through the outer cortex over the guidewire. Although many screw systems are self-tapping, distraction of fracture fragments can occur as the screw engages the far fragment. For this reason, drilling down to a depth 2 mm longer than the intended screw length can be beneficial (Fig. 20.17). Closely observe the fracture site and use the joysticks to resist any displacement that may occur with passage of the drill or screw. As the screw is advanced over the guide pin, compression and increasing resistance occur as it nears the end of the drill tunnel. Remove the guide pin and the derotation wire during the final stages of seating the screw to facilitate fracture-site compression. The proximal end of the screw must be countersunk beneath the proximal cortex. If it is prominent, replace the guide pin and change the screw out for the next shorter size.

Capitate fractures are managed using cannulated compression screw techniques similar to those already described for the scaphoid. Fixing a capitate fracture prior to a coexisting scaphoid fracture facilitates access for reduction and accurate screw placement in the proximal capitate fragment. At times, the proximal pole of the capitate may flip a full 180 degrees, with the cartilaginous dome facing distally. Reduction can be performed either using a K-wire joystick or simply with a dental pick, because the distal fragment is immobile. Once the fracture is reduced, pass the guide pin and follow the remaining fixation steps as already outlined for scaphoid fractures (Fig. 20.18).

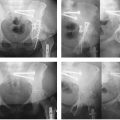
In the case of a complete lunate dislocation (Mayfield stage IV), the unreduced lunate rests in the palmar canal. As mentioned earlier, the palmar capsule may impede closed reduction. In such cases, the palmar rent in the palmar capsule may be extended through the dorsal exposure to allow the lunate to be “flipped up” back into the lunate fossa. If this cannot be accomplished through the dorsal approach, or if the patient has a progressive acute carpal tunnel syndrome, a separate palmar approach will be required.
Once the lunate rests in the lunate fossa, formal scapholunate reduction and stabilization may proceed. Again, placement of K-wire joysticks dorsally in the lunate and proximal pole of the scaphoid facilitates reduction. Typically, the lunate tends to rest in an extended position relative to the scaphoid with disruption of the scapholunate ligament. Therefore, place the joysticks in such an orientation to allow some flexion of the lunate and extension of the scaphoid with reduction. Direct observation is the best method to tell if the scapholunate joint is reduced because subtle rotational offset is difficult to determine with reliance on fluoroscopy alone. A Freer elevator placed in the radioscapholunate interval should assist in adequate visualization for this determination.
Prior to actually performing the reduction, preparations are made for accurate placement of the 0.045-inch fixation wires while avoiding injury to the sensory branches of the radial nerve and the radial artery. To determine the approximate level of skin entry for the wire, simply hold it superficial to the wound to determine the angle needed to traverse the scapholunate joint while staying out of the scaphocapitate interval (Fig. 20.19). A small stab wound is then made with a knife through the dermis only at the appropriate entry point. Use a small-tipped hemostat to spread down to the joint capsule overlying the appropriate entry level of the distal scaphoid. While holding the tips of the hemostat open, place a 14-gauge metal cannula up against the scaphoid. Use the fluoroscope to determine the correct tilt of the cannula for accurate passage across the scapholunate interval while the assisting surgeon maintains the reduction. Keeping the cannula firmly seated against bone, drill the K-wire through it across the scapholunate interval. Pass the wire far enough for good purchase in the lunate while avoiding penetration into the radiocarpal joint. Pass a second 0.045-inch K-wire across the joint into the lunate for better stability using the same method. A third wire is then placed across the scaphocapitate joint.

Although most hand surgeons employ K-wires for scapholunate stabilization, an alternative method is temporary scapholunate compression screw fixation. Proposed advantages over K-wires include increased strength of repair and decreased risk of pin-site infection or migration. The technique entails placement of the guide pin for a 3.0- to 3.5-mm compression screw across the center scapholunate articulation in the lateral projection, taking care to avoid violating the midcarpal or radiocarpal joints. While the sagittal alignment is maintained with K-wire joysticks, the screw is advanced across the scapholunate joint and compresses the gap (Fig. 20.20). The ligament is then repaired with suture anchors, as described above. The screw is subsequently removed after 3 or 4 months. Results with this method have been similar to those seen with K-wire fixation.9,10

Now that the scapholunate interval is reduced and stabilized, repair the ligament using small anchors with 4-0 braided suture. Usually, the tear is more off one bone, while the majority of the ligament remains attached to the other. Use a small rongeur or curet to debride a small area of the bone in preparation for ligament reattachment (Fig. 20.21). Two or three anchors are placed in the proximal pole of the bone to which the ligament is to be repaired. The attached 4-0 suture is then passed through the ligament in a horizontal mattress fashion and tied down to secure the ligament to the bed of prepared bone (Fig. 20.22).


In some instances (often seen in more subacute presentations several weeks following injury), there may not be sufficient ligament tissue to offer a substantial primary repair. Many authors have advocated dorsal capsulodesis using a flap of proximally based dorsal capsule to augment the ligament repair.11 The distal portion of the tongue of the dorsal capsule is attached to the dorsal portion of the distal pole of the scaphoid (after it has been restored to anatomic position relative to the lunate) through the use of either drill tunnels or suture anchors. Following the 8 to 12 weeks of immobilization, this may help resist the tendency of the scaphoid to drift into flexion, should a weak scapholunate ligament repair be inadequate.
As already mentioned, some surgeons perform pin fixation alone of the lunotriquetral joint without performing formal repair of the ligament. Should ligament repair be desired, it can be done using the steps outlined earlier for repair of the scapholunate ligament. The triquetrum tends to rest slightly dorsal relative to the lunate with tears of this ligament, and dorsal pressure against the triquetrum for reduction should be performed when passing the wires across the joint. Again, use of the 14-gauge metal sleeve will minimize the risk of injury to the dorsal sensory branches of the ulnar nerve. If a reparable flap of the lunotriquetral ligament is visible, a direct repair using small suture anchors or bone tunnels may be utilized. However, recall that the dorsal portion of the lunotriquetral ligament is less substantial than the palmar portion. Therefore, some surgeons employ a separate palmar approach through the floor of the carpal canal to perform a direct palmar ligamentous repair.
Once the carpus has been stabilized, the pins are either cut short beneath the skin or bent down and capped outside the skin. As we prefer to leave the pins in place for at least 2 months, leaving them buried under the skin may minimize the likelihood for infection. Should they be cut beneath the skin, ensure they are cut short enough. Prominent pins eventually erode through and result in an infection underneath the cast.
If a radial styloid fracture is large enough to involve the radioscaphocapitate ligament or to create a significant deformity in the scaphoid fossa, it should be reduced and stabilized. If a transverse skin incision has been employed to address the carpal instability, it can be extended radially to expose the radial styloid underneath the first dorsal compartment tendons. If a longitudinal dorsal incision was used, make a second longitudinal incision centered over the radial styloid (Fig. 20.23). With either approach, take a moment to identify and gently retract the sensory branches of the radial nerve. Failure to do so will result in a painful neuroma or an anesthetic area on the dorsoradial aspect of the hand that consumes the patient′s attention despite a perhaps otherwise good outcome. Once nerve branches have been identified and reflected, divide the extensor retinaculum over the first dorsal compartment and reflect the underlying tendons to expose the radial styloid and brachioradialis insertion. The radial artery can be found lying deep to the tendons just beyond the radial styloid and should be protected. Although the styloid fragment may appear reduced along the radial metaphysis, it is important to confirm reduction of the joint surface by fluoroscopy or direct visualization. Subtle rotational deformities of the fragment often result in significant joint incongruity. Once reduction is confirmed, a compression screw is placed across the fracture into the radial metaphysis. A cannulated 4.0-mm screw or regular 3.5-mm screw placed over a washer using lag technique is sufficient, although K-wires or radial column plates may be used. A cannulated compression screw also works quite well and avoids the issue of prominent screw heads (Fig. 20.24).


Clearly, not all ulnar styloid fractures need to be stabilized. Only those ulnar styloid fragments that encompass the deep insertion of the triangular fibrocartilage require fixation (Fig. 20.25). This may be assessed by simply stressing the distal radioulnar joint in the sagittal plane after other distal radius and carpal injuries have been stabilized. If the joint is unstable, then the ulnar styloid should be fixed to restore the insertion of the triangular fibrocartilage into the ulna. We find that a combination K-wire and tension-band technique provides stable, low-profile fixation.

The approach to the distal ulna is made through a longitudinal incision beginning ~ 4 cm proximal to the styloid along the ulnar subcutaneous border. Distally, the dorsal sensory branch of the ulnar nerve traverses the incision around the tip of the styloid and should be protected. Release the ulnar extent of the extensor retinaculum to expose the fracture. Use either a knife or an elevator to expose the ulnar aspect of the cortex for a distance of 2 to 3 cm proximally. After obtaining the reduction with a dental pick, pass a 0.045-inch K-wire through the tip of the styloid into the cortex of the ulnar metaphysis proximally (Fig. 20.26). A 24-gauge K-wire is used to establish the tension-band construct. Passing the cerclage wire around the base of the styloid is facilitated by using a bent 18-gauge needle passed around the base. The wire is then inserted into the protruding sharp tip of the needle and pushed through it. If the wire becomes incarcerated and will not pass through the needle, simply withdrawing the needle will usually result in the wire being pulled through with it. After crossing the wire over the cortex of the ulna, pass it through a transverse drill hole in the ulna 2 to 3 cm proximal to the styloid. The cerclage wire is then twisted together to provide compression of the fracture through a tension-band effect. The K-wire is bent, cut, and advanced down to the tip of the styloid (Fig. 20.27).


A palmar approach may be required for an urgent carpal tunnel release or reduction of an incarcerated lunate dislocation. This is performed through a more extensile incision than that traditionally used for a routine carpal tunnel release. Beginning at the level of Kaplan′s cardinal line (Fig. 20.28), the incision courses proximally between the thenar crease and the hook of the hamate. The incision end ~ 2 cm proximal to the wrist flexion crease. Dissection proceeds down to the level of the transverse carpal ligament, which is divided sharply along its full length while avoiding injury to the underlying median nerve. Following evacuation of the hematoma, an incarcerated lunate may be exposed by retracting the carpal canal contents. Avoid placing retractors along the radial aspect of the median nerve with this maneuver, as this may result in injury to the recurrent motor branch. If the lunate is stuck in the canal, make a relaxing incision in the palmar capsule to enable reduction back into the lunate fossa.

Tips and Tricks
Palpation of the entire wrist (distal radius, distal radioulnar joint [DRUJ], radial and ulnar styloids, specific carpal bones and joints) with attention to each component is the mainstay of a competent assessment for perilunate injuries. Even in the acute setting, patients are more focally tender over specific fracture or ligament injuries than elsewhere in the wrist. This is the key to not missing more subtle injury presentations.
Traction radiographs in the acute setting are helpful to delineate the injury pattern.
A clenched-fist view with ulnar deviation can reveal an otherwise normal-appearing scapholunate gap or scaphoid fracture on plain film.
Carefully evaluate the joint for the presence of bone or loose cartilage fragments that could become problematic later.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree