Fig. 15.1
Chest x-ray of the patient showing the pacemaker in situ and the approximate position of the breast cancer
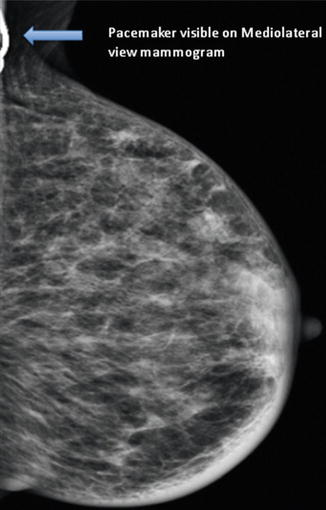
Fig. 15.2
Mammogram (mediolateral view) showing the pacemaker. The breast cancer was mammographically occult
After discussion at the MDT meeting, we recommended wide local excision and sentinel node biopsy, and in view of the size of the tumour and the presence of the pacemaker, it was also decided to offer the patient IORT using the TARGIT technique.
Prior to surgery, the details of the pacemaker device were obtained from the implanting hospital, and the distance from the tumour to the device measured at an assessment session. During surgery, after harvesting the sentinel node and wide local excision, IORT using the INTRABEAM device (50 kV) was performed. A 3-cm-diameter applicator was used, delivering approximately 20 Gy at the surface of the breast tissue in direct contact with the applicator, and 6 Gy at 1 cm from the surface, for the duration of 26 min. During the surgery a packet of thermoluminescent dosimeters (TLD) was placed on the edge of the device closest to the X-ray source and the distance between the applicator shaft of the INTRABEAM and the pacemaker was recorded. The measured reading was converted to dose using a batch calibration value corrected for supralinearity. The average reading of the TLD packet was 0.08 Gy.
The patient tolerated the procedure very well; there was no malfunction of the pacemaker during the surgery and IORT and the patient was discharged home the following day. Pacemaker function was tested by a cardiology technician before and after treatment.
Histology confirmed a 14-mm grade 2 invasive ductal carcinoma which was node negative and oestrogen receptor positive with a Quick score of 8, progesterone receptor positive with a Quick score of 8 and HER2 negative. She was advised an aromatase inhibitor as adjuvant treatment.
15.4.1 Discussion
The position of this patient’s pacemaker was such that it would have been in the tangential whole breast radiotherapy fields and would have thus received the full dose. There is a risk that ionising irradiation can interfere with modern CMOS (complementary metal oxide semiconductor) equipped cardiac pacemakers (Hudson et al. 2001), resulting in their failure. The option with this patient was to reposition the pacemaker and give standard EBRT or to treat the tumour bed with IORT, allowing the dose to the pacemaker to be kept low.
In 1994, the American Association of Physicists in Medicine (AAPM) stated that a cardiac pacemaker can fail at radiotherapy (RT) doses as low as 10 Gy and even 2 Gy could lead to significant functional changes (Marbach et al. 1994). This resulted in the “AAPM guideline for pacemakers in radiation oncology”, which suggested that the dose at the pacemaker should be limited to 2.0 Gy (Marbach et al. 1994).
Nowadays, modern pacemakers are equipped with CMOS circuitry and RAM (random access memory). These electronic elements facilitate programming of devices (e.g. holding the current information about frequency threshold, modes of pacing and sensing) and improve reliability and energy consumption. Nevertheless, an increase in IR sensitivity has been described as well. Several cases have been reported where the threshold programming was deleted or the devices failed at low doses (overview at Hudson et al. 2001). On the other hand, no manufacturer has quoted a safe minimum radiation dose.
At present, very little is known about direct and scatter irradiation effects, especially concerning the newer pacemakers equipped with modern CMOS. Manufacturers provide estimates for their pacemaker models regarding to which dose a device can be irradiated (St. Jude 20–30 Gy (Rhythm Management Division 2005), Medtronic 5 Gy (CRDM Technical Services 2008), Guidant n.n. (European Technical Services 2004)) The wide range of manufacturer-stated acceptable radiotherapy doses and in vitro data generated since the 1994 AAPM guideline for radiotherapy (Marbach et al. 1994), showing that PM can fail at any dose (Mouton et al. 2002), represent the fragile situation at which radiotherapy operates in regard to modern CMOS-equipped pacemakers.
Therefore, at least for EBRT, in cases where the pacemaker would be close to treatment fields, adjustments (encompassing modification of the radiotherapy field size and shape, moving the pacemaker surgically out of the field, withholding radiotherapy from the patient or considering IORT if appropriate) to the treatment planning procedure might become necessary.
15.5 Case 5: Randomisation into the TARGIT-A Trial
A 69-year-old woman attended for routine breast screening and was found on mammography to have a 22-mm spiculated mass with microcalcification in the left upper outer quadrant (M5). Ultrasound revealed a hypoechoic lesion with normal lymph nodes (U5). A core biopsy diagnosed a grade 2 ductal carcinoma that was oestrogen receptor positive (Quick score 8), progesterone receptor positive (Quick score 8) and HER2 negative (B5). MRI scan showed a unifocal, unicentric mass 16 mm in size with type II enhancement and no lymphadenopathy.
Her case was discussed in the MDT meeting and a recommendation for wire-guided localisation of the lesion was made prior to wide local excision and sentinel node sampling. The patient met the criteria for entry into the TARGIT trial. This was discussed with her prior to surgery and she agreed to participate in the trial and was randomised to the IORT arm. At the time of surgery a dose of 6 Gy at 1 cm from the applicator surface was delivered. OSNA testing of the sentinel node was positive and the surgeon therefore went ahead with an axillary clearance.
Following the surgery her case was again discussed at the multidisciplinary meeting. The final histology confirmed the presence of a 17-mm grade 3 ductal carcinoma with associated DCIS; all margins were clear and 3 out of 11 lymph nodes were positive. The MDT meeting therefore recommended staging with bone scan and CT of the chest, abdomen and pelvis, followed, if clear, by chemotherapy, radiation treatment to the whole breast and endocrine treatment.
15.5.1 Discussion
At initial presentation, imaging and biopsy suggested we were dealing with a low-risk breast cancer. It was therefore reasonable to offer the patient entry into the TARGIT trial, which compares IORT with standard radiotherapy consisting of whole breast irradiation followed by a boost to the tumour site (Vaidya et al. 2010). However, following surgery this lady was found to be node positive and therefore at higher risk for both local and systemic recurrence. This being the case, the above recommendation was made in order to try and reduce the risk of recurrence. Results from the TARGIT trial revealed that following IORT about 15 % of patients went on to receive whole breast irradiation because of upstaging of the disease. This patient had 40.05 Gy in 15 fractions to the left breast.
15.6 Case 6: Previous Radiotherapy in the Treatment Area
A 63-year-old lady presented with a lump in her right breast. On examination she had a 10-mm lump in the upper outer quadrant of the right breast. Mammogram and ultrasound findings were diagnostic of breast cancer (M5, U5). Core biopsy revealed a grade 1 invasive ductal carcinoma that was oestrogen receptor positive (Quick score 8), progesterone receptor positive (Quick score 8) and HER2 negative.
She had been treated 12 years earlier for non-Hodgkin’s lymphoma centred on the left neck. She initially received CHOP × 6, ESHAP × 2 mini beam but a year later developed resistant diffuse B cell lymphoma which was a presumed transformation from follicular lymphoma. She went on to have full-dose BEAM with autologous bone marrow transplant followed by radiotherapy to the mediastinum and neck.
She was discussed in the MDT meeting and the recommendation was for mastectomy because the right breast would have received a significant radiation dose from the mediastinal radiation fields employed 12 years earlier for the treatment of her lymphoma. However, the patient was adamant that she wished to undergo breast-conserving surgery. IORT off-trial was offered to her on the understanding that if she was found to have high-risk disease following the wide local excision and sentinel lymph node sampling, mastectomy would have to be considered.
She underwent right wire-guided wide local excision and sentinel node sampling with IORT. At the time of surgery a dose of 6 Gy at 1 cm from the applicator surface was delivered. The histology confirmed a 14.4-mm grade 1 invasive ductal carcinoma with clear margins but an incidental finding of follicular lymphoma in the sentinel node. The MDT recommended Arimidex for 5 years and referral to the haematologist with regard to her follicular cell lymphoma.
15.6.1 Discussion
Radiation-induced secondary malignancies can occur many years after patients have received treatment. They commonly occur within the radiation field. There is a growing amount of data for patients who receive chemotherapy and radiotherapy for Hodgkin’s disease, where an increase in second solid tumours has been observed, especially cancers of the lung, breast, thyroid, bone/soft tissue, stomach, oesophagus, colon and rectum, uterine cervix, head and neck, and mesothelioma. These tumours occur primarily after radiation therapy or with combined modality treatment, and approximately 75 % occur within radiation ports. At a 15-year follow-up, the risk of second solid tumours is approximately 13 %.
In a case control study of 106 patients who developed breast cancer after therapy for Hodgkin’s disease, cumulative absolute risks for developing breast cancer were calculated as a function of radiation therapy dose and the use of chemotherapy. With a 30-year follow-up, cumulative absolute risks of breast cancer with exposure to radiation range from 8.5 to 39.6 %, depending on the age at diagnosis. The younger the age at treatment, the higher is the incidence.
There are a number of patients in whom standard breast radiotherapy cannot be delivered because of previous radiotherapy in that area for other malignancies such as lymphoma and oesophageal and lung cancer. In these cases the standard recommendation would be mastectomy, thus avoiding radiotherapy. In patients with low-risk breast cancer, IORT at the time of surgery offers the chance of undergoing breast-conserving surgery.
15.7 Case 7: Inclusion into Kypho-IORT Dose Escalation Study
A 60-year-old female patient with osseous metastasizing breast cancer under tamoxifen, trastuzumab and denosumab presented with newly diagnosed progressive metastases of the 9th and 10th thoracic vertebrae (Fig. 15.3). The remaining osseous metastases were stable or regressive. There were no visceral or lymphatic metastases. On clinical examination the patient had a Karnofsky Index of 100 %, without any back pain. The patient had a strong therapy wish.
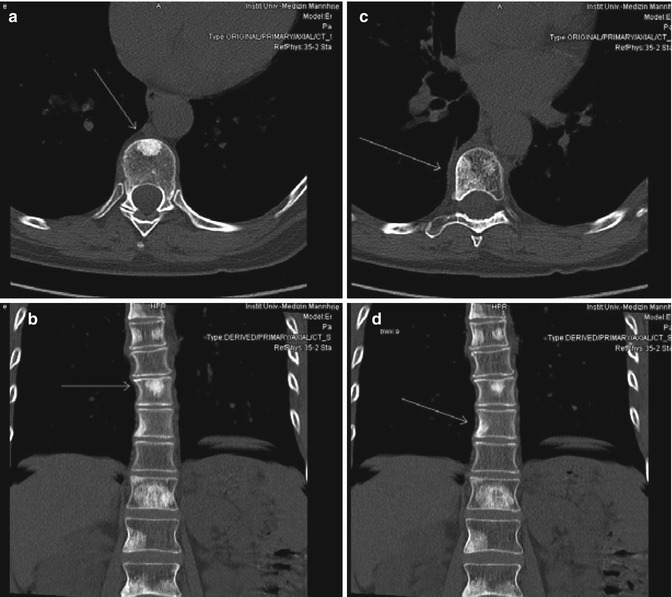

Fig. 15.3
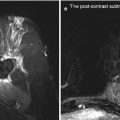
Postoperative follow-up T1-weighted MRI demonstrating enhancement in the operative site with compensatory dilatation of the left occipital horn of the lateral ventricle
The initial diagnosis of a receptor-positive invasive ductal carcinoma [pT1c pN3a (12/15) L1 V0 R0 M1] of the left breast was made in May 2011. After breast-conserving surgery with axillary dissection, the patient received whole-breast radiotherapy including the axillary lymph nodes with boost (50 + 16 Gy) followed by systemic therapy using trastuzumab, Aromasin and bisphosphonates. Simultaneous to the whole breast radiotherapy, the patient received a palliative irradiation of the right hip (40/2 Gy). The first progressive osseous metastases were diagnosed in June 2012 during routine follow-up and palliative irradiation of C3-T1, T5-T8 and T11-L4 (30/3 Gy) was initiated. Moreover, endocrine therapy was switched from Aromasin to tamoxifen, and denosumab was started instead of bisphosphonates.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree