Central Nervous System Infections
Nathaniel A. Chuang
Walter L. Olsen
Neuroimaging is an important tool used in the evaluation and treatment of infections of the central nervous system (CNS). These infections frequently have dire neurologic consequences, and their early diagnosis and management, with the aid of CT and MR in particular, are crucial. Prior to the widespread availability of CT, pyogenic abscesses of the brain carried a 30% to 70% mortality rate. The mortality rate has since dropped to less than 5%, largely because of the ability of neuroimaging to accurately diagnose and localize abscesses and monitor the efficacy of appropriate interventions. MR is usually the imaging modality of choice for CNS infections because of its improved sensitivity and specificity compared to CT. However, CT can be preferred for unstable and/or uncooperative patients because it allows much shorter imaging times and easier patient monitoring.
Congenital Infections
Congenital infections of the fetal and neonatal brain are commonly referred to as the group of TORCH infections, which include toxoplasmosis, other infections (such as syphilis and varicella), rubella, cytomegalovirus, and herpes simplex (and HIV). The pathogens causing these infections can be transmitted transplacentally in utero or during the birth process. These infections often result in significant brain injury, and congenital brain malformations are more frequently seen with earlier onset of infections in utero due to disruption of normal CNS development during fetal gestation.
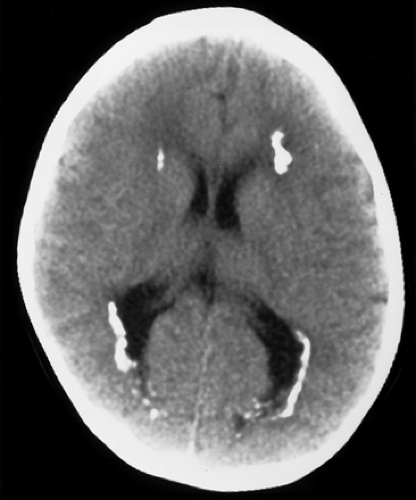
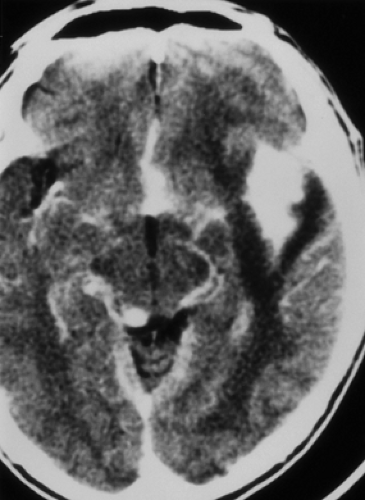
Cytomegalovirus (CMV) is a member of the herpes family of viruses and is the most common cause of congenital CNS infection. In utero transmission occurs hematogenously during viral reactivation in seropositive pregnant women (CMV-seropositivity in different populations worldwide ranges between 40% and 100%) or as primary infection during pregnancy. Maternal CMV infection results in transplacental transmission to the fetus in 30% to 50% of cases and symptomatic disease in 5%. Postnatal infection can occur via viral shedding in breast milk. Symptomatic neonates may have hepatosplenomegaly, jaundice, cerebral involvement (psychomotor retardation), chorioretinitis, and deafness. The virus preferentially multiplies along the ependyma and germinal matrix, resulting in a periventricular pattern of injury and development of dystrophic calcifications. Obstetrical and neonatal cranial US can demonstrate hypoechoic periventricular ring-like zones, and the subsequent characteristic hyperechoic periventricular calcifications. CT without contrast best depicts these periventricular calcifications (Fig. 6.1). There are usually no calcifications of the basal ganglia or cortex as is seen in congenital toxoplasmosis. Loss of periventricular white matter results in ventriculomegaly and microcephaly. Infections during the first trimester can result in neuronal migrational anomalies, such as agyria, cortical dysplasia and heterotopia, which are better shown by MR. Delayed myelination and cerebellar hypoplasia are also common findings. CNS malformations are less common in patients infected later during gestation but delayed myelination and periventricular white matter lesions are still seen.
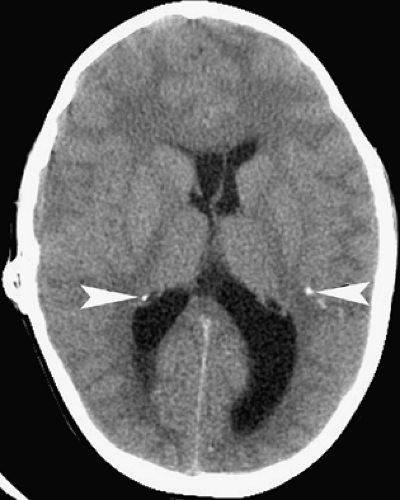
Toxoplasmosis follows CMV infections in frequency among congenital CNS infections, and is caused by the parasitic protozoan Toxoplasma gondii, which occurs worldwide. Congenital infection results from hematogenous spread after a pregnant woman eats undercooked meat or is exposed to cat feces, both of which can harbor viable oocysts. A necrotizing encephalitis of the fetal brain ensues, causing severe destruction, especially during the first two trimesters of gestational, but typically no developmental malformations. The infant is usually born with microcephaly, chorioretinitis, and mental retardation. Imaging studies reveal atrophy, dilated ventricles, and dystrophic calcifications (Fig. 6.2). The calcifications are scattered in the white matter, basal ganglia, and cortex. This is in distinction to the primarily periventricular calcifications observed in congenital CMV infection.
Herpes simplex encephalitis in neonates most often results from infection during descent through the birth canal when the mother has a genital infection with herpes virus type 2. Occasionally, there is transplacental transmission before delivery, but this usually results in spontaneous abortion. CNS infection causes a diffuse encephalitis with infarction, which is either fatal or has severe neurologic consequences.
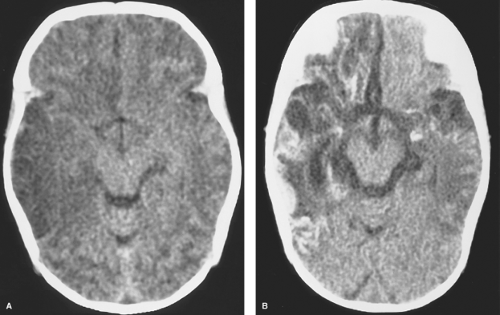
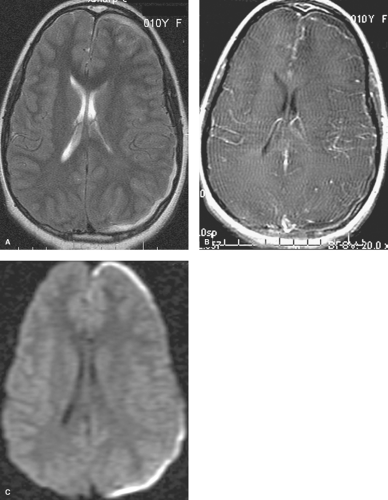
The infant typically presents with a fever, rash, lethargy, and seizures in the first several weeks of life. CSF analysis reveals pleocytosis, increased protein, and decreased glucose. If the patient survives, varying degrees of microcephaly, mental retardation, microphthalmia, enlarged ventricles, intracranial calcifications, and multicystic encephalomalacia may occur. Early in the course of the encephalitis, cranial US will show areas of increased parenchymal echogenicity. CT may demonstrate diffuse brain swelling or bilateral patchy areas of hypodensity in the cerebral white matter and cortex, with relative sparing of the basal ganglia, thalami, and posterior fossa structures (Fig. 6.3A). These hypodense lesions correspond to areas of T2-hyperintensity on MR and progress to areas of necrosis and cystic encephalomalacia. Associated hemorrhage, calcifications, and meningeal and patchy parenchymal enhancement can be seen with both CT and MR (Fig. 6.3B).
The infant typically presents with a fever, rash, lethargy, and seizures in the first several weeks of life. CSF analysis reveals pleocytosis, increased protein, and decreased glucose. If the patient survives, varying degrees of microcephaly, mental retardation, microphthalmia, enlarged ventricles, intracranial calcifications, and multicystic encephalomalacia may occur. Early in the course of the encephalitis, cranial US will show areas of increased parenchymal echogenicity. CT may demonstrate diffuse brain swelling or bilateral patchy areas of hypodensity in the cerebral white matter and cortex, with relative sparing of the basal ganglia, thalami, and posterior fossa structures (Fig. 6.3A). These hypodense lesions correspond to areas of T2-hyperintensity on MR and progress to areas of necrosis and cystic encephalomalacia. Associated hemorrhage, calcifications, and meningeal and patchy parenchymal enhancement can be seen with both CT and MR (Fig. 6.3B).
Congenital HIV. Infection with HIV can occur transplacentally during childbirth and postnatally via breast feeding. Affected infants are more susceptible to respiratory infections and diarrhea and can present with encephalopathy, developmental delay, and failure to thrive. The opportunistic infections and neoplasms seen in adults with acquired immunodeficiency syndrome (AIDS) are not usually observed in young children. HIV encephalitis primarily affects white matter and basal ganglia resulting in diffuse cerebral volume loss. Symmetric calcifications in the basal ganglia but especially the globi pallidi are best seen with CT, whereas MR allows better demonstration of T2-hyperintense white matter abnormalities. Subtle enhancement of the basal ganglia can occasionally be detected. In some cases, MR angiography (MRA) may reveal an associated vasculopathy with fusiform dilation and ectasia of the intracranial arteries.
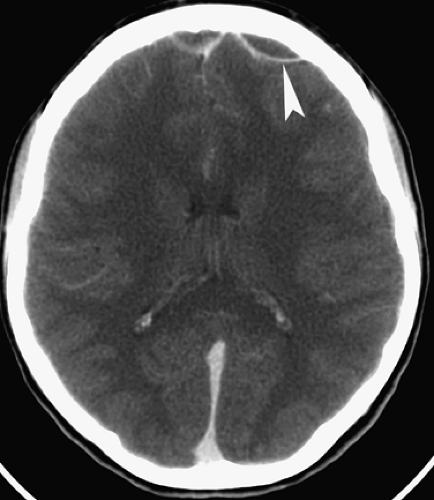
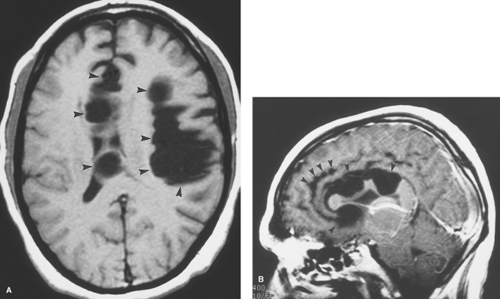
Rubella was once a devastating fetal viral infection but is now very uncommon because of widespread immunization of women before their child-bearing age. Transplacental transmission takes places during maternal infection with the worst consequences arising from first trimester infections causing diffuse meningoencephalitis, brain infarction, and necrosis. Infants who survive severe infections present with microcephaly, ocular abnormalities, and deafness. CT reveals dystrophic calcifications in the deep gray nuclei and cortex (Fig. 6.4), whereas MR better demonstrates infarcts, white matter volume loss, and, occasionally, delayed myelination.
Extra-Axial Infections
Subdural and Epidural Infections
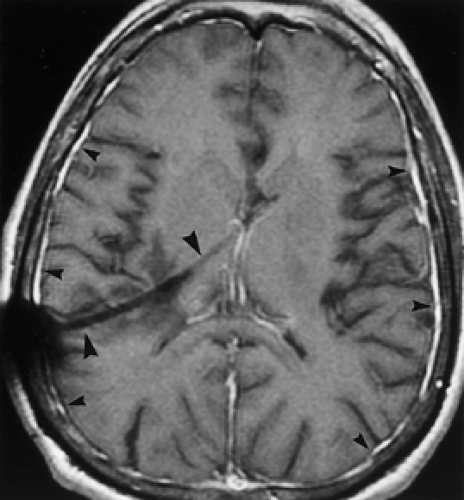
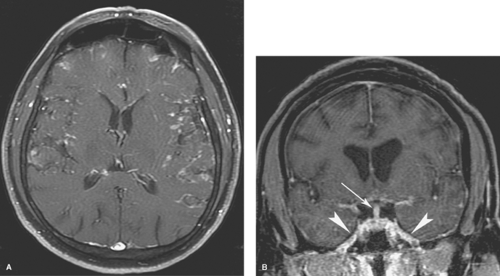
Extra-axial pyogenic infections can involve the epidural or subdural spaces. Both epidural and subdural abscesses or empyemas may result from paranasal sinusitis, otomastoiditis, orbital infections, penetrating injuries, surgery, or superinfection of preexisting extra-axial collections. CT and MR scans show an extra-axial collection with increased density (Fig. 6.5) or increased T1 and T2 signal intensity compared to CSF. The margins of the collection usually enhance smoothly with contrast. MR is more sensitive than CT for both epidural and subdural empyemas because the multiplanar capability of MR alleviates the problem of partial volume averaging with the calvarium on CT. Cranial US in infants can demonstrate heterogeneous echogenic extra-axial collections as well as hyperechoic material in the subarachnoid space if the child also has meningitis. Epidural empyemas are generally confined by dural attachments and this prevents rapid expansion of epidural abscesses and account for their lentiform shape and convex inner margins. However, subdural empyemas can spread more easily through the subdural space and be more acutely life-threatening (Fig. 6.6A, B), thus requiring rapid neurosurgical intervention. Subjacent cerebritis may develop with both entities. Cortical venous thrombosis resulting in venous infarcts is
a common result of these infections, and MR and MR venography (MRV) allow easier detection of venous thrombosis and venous infarcts. Evaluation for adjacent sinusitis or skull abnormalities is also required. Frontal sinusitis in children can be complicated by osteomyelitis, with subperiosteal, epidural, or subdural abscesses. This infection is known as Pott puffy tumor. Subdural empyemas can be hyperintense on diffusion-weighted imaging (DWI) thus allowing them to be distinguished from subdural effusions (Fig. 6.6C), which can also enhance mildly. Subdural hygromas are identical to CSF in density and signal intensity and do not enhance.
a common result of these infections, and MR and MR venography (MRV) allow easier detection of venous thrombosis and venous infarcts. Evaluation for adjacent sinusitis or skull abnormalities is also required. Frontal sinusitis in children can be complicated by osteomyelitis, with subperiosteal, epidural, or subdural abscesses. This infection is known as Pott puffy tumor. Subdural empyemas can be hyperintense on diffusion-weighted imaging (DWI) thus allowing them to be distinguished from subdural effusions (Fig. 6.6C), which can also enhance mildly. Subdural hygromas are identical to CSF in density and signal intensity and do not enhance.
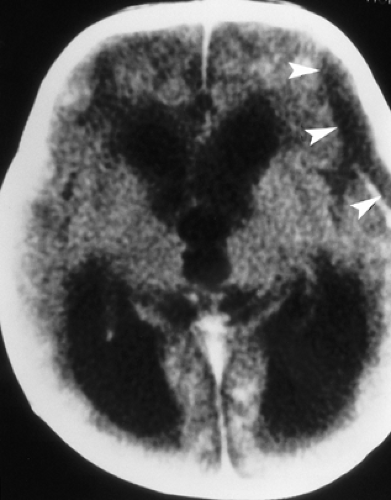
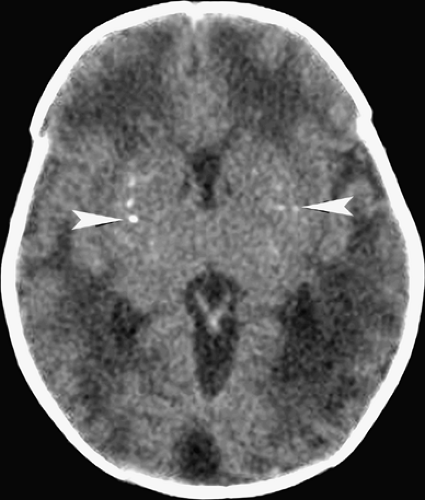 Figure 6.4. Congenital Rubella. Nonenhanced CT image in this neonate demonstrates multiple punctate hyperdense calcifications in the bilateral basal ganglia (arrowheads) and hypodense white matter. |
Mild, smooth dural, or meningeal enhancement may be seen after craniotomies and in patients with ventriculostomy catheters, especially with MR (Fig. 6.7). This enhancement can persist for years and should be considered benign in this clinical setting. It most likely reflects a chemical meningitis resulting from perioperative hemorrhage and/or dural scarring. Intracranial hypotension from a spontaneous or iatrogenic CSF leak (including recent lumbar puncture) can also result in smooth symmetric dural enhancement both intracranially and along the spinal canal.
Meningitis
Meningitis can be caused by bacteria, mycobacteria, fungi, parasites, or viruses. Bacterial meningitis is caused by
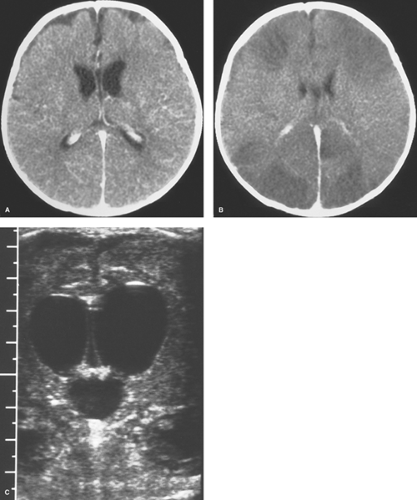
Haemophilus influenzae (in children), Neisseria meningitidis (in teenagers and young adults), and Streptococcus pneumoniae (in older adults) in more than 80% of cases. Meningitis caused by group B streptococcus and Escherichia coli occurs in neonates, whereas that caused by Citrobacter is seen commonly in premature newborns. The bacteria most commonly enter the meninges during systemic bacteremia but can spread directly from infected sinuses or after surgery or trauma. Patients present with a relatively acute onset of fever, neck stiffness, irritability, and headache, followed by a decline in the mental status. CSF studies are usually diagnostic, and CT scans performed in the emergency setting are frequently normal (Fig. 6.8A). The inflammatory exudate caused by the meningitis may produce high density on CT and hyperintensity on FLAIR MR sequences within the subarachnoid spaces and ventricles. Other differential diagnostic considerations include ruptured aneurysm with subarachnoid hemorrhage, leptomeningeal metastases, neurosarcoidosis, and lymphoma. Diffuse cerebral edema is sometimes seen (Fig. 6.8B). If contrast is given, meningeal enhancement can range from being absent or subtle to very thick and extensive.
Haemophilus influenzae (in children), Neisseria meningitidis (in teenagers and young adults), and Streptococcus pneumoniae (in older adults) in more than 80% of cases. Meningitis caused by group B streptococcus and Escherichia coli occurs in neonates, whereas that caused by Citrobacter is seen commonly in premature newborns. The bacteria most commonly enter the meninges during systemic bacteremia but can spread directly from infected sinuses or after surgery or trauma. Patients present with a relatively acute onset of fever, neck stiffness, irritability, and headache, followed by a decline in the mental status. CSF studies are usually diagnostic, and CT scans performed in the emergency setting are frequently normal (Fig. 6.8A). The inflammatory exudate caused by the meningitis may produce high density on CT and hyperintensity on FLAIR MR sequences within the subarachnoid spaces and ventricles. Other differential diagnostic considerations include ruptured aneurysm with subarachnoid hemorrhage, leptomeningeal metastases, neurosarcoidosis, and lymphoma. Diffuse cerebral edema is sometimes seen (Fig. 6.8B). If contrast is given, meningeal enhancement can range from being absent or subtle to very thick and extensive.
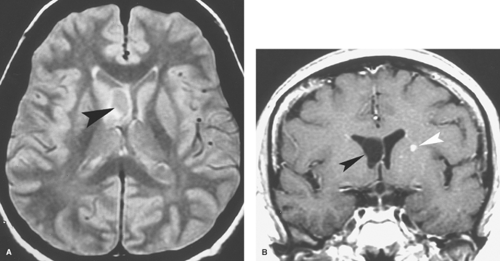
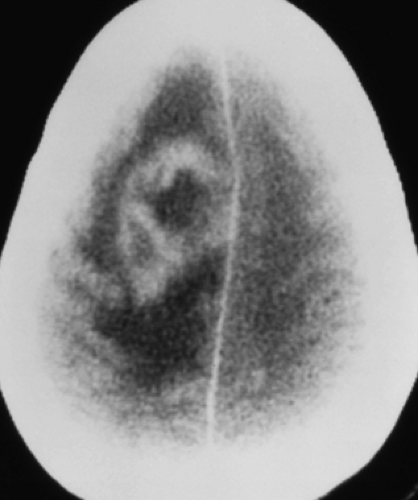
Neuroimaging is perhaps used more importantly later in the course of meningitis when there are suspected complications such as hydrocephalus, cerebritis or abscess (to be discussed later), arterial or venous infarction, subdural effusion or empyema, and herniation. Communicating hydrocephalus is more typical than the noncommunicating type and reflects impaired CSF resorption by arachnoid granulations. Assessment of arterial and venous infarction with MR can be done with a combination of DWI, MRA, and MRV. Contrast-enhanced CT angiography (CTA) and CT venography (CTV) are also helpful but are associated with increased radiation exposure. Subdural effusions may be seen in infants, especially in meningitis caused by H. influenzae. Subdural effusions appear as thin collections along the surface of the brain and are isodense on CT and isointense with CSF on MR (Fig. 6.9) as well as may show mild enhancement with contrast agents. These sterile effusions can also be identified with cranial sonography in infants. Echogenic sulci, ventriculomegaly, and abnormal parenchymal echogenicity are visualized by US in infants with bacterial meningitis (Fig. 6.8C).
Tuberculous meningitis is the most common form of CNS tuberculosis. It is usually caused by Mycobacterium tuberculosis, but can rarely be caused by atypical mycobacteria, such as M. avium-intracellulare. Tuberculous meningitis occurs in all age groups but particularly in children and the elderly. Patients with AIDS, prisoners, and immigrants from regions with endemic tuberculosis (TB) are also affected disproportionately. Approximately 5% to 10% of patients with TB develop CNS disease. The disease spreads to the meninges hematogenously from the lungs, but the chest radiograph is normal in 40% to 75% of patients. The tuberculin skin test can be deceivingly negative. Clinically, there is usually a subacute or insidious onset of headache, malaise, weakness, apathy, or focal neurologic findings. CSF should demonstrate pleocytosis, elevated protein, and markedly reduced glucose levels. Mycobacterial cultures of CSF may be negative or take weeks to confirm an infection, and polymerase chain reaction (PCR) studies may be more sensitive. Imaging studies will show enhancing, thickened meninges, especially along the basal cisterns (Fig. 6.10), corresponding to a thick gelatinous inflammatory exudate. In contrast, meningeal enhancement in bacterial meningitis is usually more peripherally distributed and less thick when compared to tuberculous and other granulomatous meningitides. The differential diagnosis of tuberculous meningitis includes fungal meningitis, racemose cysticercosis, neurosarcoidosis, and carcinomatous meningitis.
Tuberculous meningitis can present with concomitant infection of the brain parenchyma in a miliary pattern or with larger tuberculomas or abscesses (discussed later in detail). Frequent complications include hydrocephalus or infarcts. The inflammatory exudate in the basal cisterns may extend along perivascular spaces, causing an arteritis with irregular narrowing or occlusion of vessels, and infarcts occur most commonly along the distribution of the lenticulostriate and thalamoperforator arteries and in the deep gray nuclei. MRA can be helpful.
Fungal meningitis usually causes thick meningeal enhancement in the basal cisterns, in a manner similar to tuberculous meningitis (Fig. 6.11). However, in cases of cryptococcal meningitis, the degree of enhancement varies with the immunocompetence of the patient. Hydrocephalus is common, but infarcts and extension of fungal infection into the brain substance occur less often than with tuberculous or pyogenic meningitis (except in cases of aspergillosis and mucormycosis). Fungal infections of the brain parenchyma will be discussed in more detail subsequently.
Meningobasal or racemose cysticercosis occurs when the larvae of the pork tapeworm, Taenia solium, infest the subarachnoid space, especially the basal cisterns. (Parenchymal neurocysticercosis will be discussed later.) The larval cysts may grow in grape-like clusters (Latin translation of “clusters” is “racemose”) or conform to the shape of the involved cisterns. These cystic lesions are isodense on CT and isointense on MR to CSF (Fig. 6.12). No mural nodules (i.e., parasitic scolex) or calcifications are seen, but mural enhancement of the cysts or diffuse meningeal enhancement can be observed. Hydrocephalus is often present.
Intraventricular cysticercosis can be difficult to detect by CT and MR since the cysts are usually isodense and isointense to CSF. Subtle signal changes (especially on proton-density weighted and fluid-attenuated inversion recovery [FLAIR] sequences) and the lack of CSF pulsations within the cyst makes them more visible on MR than on CT (Fig. 6.13). Enhancement may or may not be present, depending on the stage of disease, similar to the parenchymal form. A mural scolex can often be seen within these cysts. Cysts may obstruct the foramen of Monro, the Sylvian aqueduct, or the third and fourth ventricles, resulting in hydrocephalus. Death may result from acute hydrocephalus, and ventriculitis follows cyst rupture.
Viral meningitis is caused most commonly by the enteroviruses but can also be caused by mumps virus, Epstein-Barr virus (EBV), togaviruses, lymphocytic choriomeningitis virus, and HIV. Patients usually present with a flu-like illness, fever, headaches, and nuchal rigidity. Most patients do not require treatment and neurologic deficits are uncommon unless infection progresses to encephalitis. Neuroimaging studies are typically normal but mild meningeal enhancement can occur.
Sarcoidosis is a noninfectious granulomatous disease of unclear etiology involving the CNS in up to 14% of patients at autopsy. Only a minority of patients present with neurologic signs or symptoms, such as headaches, cranial neuropathies, pituitary dysfunction, seizures, or other focal neurologic deficits. Aside from biopsy, confirming increased serum and CSF levels of angiotensin-converting enzyme (ACE) and pulmonary involvement are helpful for diagnosis. Neurosarcoidosis
primarily affects the leptomeninges, and abnormal leptomeningeal and dural enhancement can be seen with both CT and MR (Fig. 6.14A). Thickening and enhancement of the cranial nerves and the hypothalamic-pituitary axis are not uncommon (Fig. 6.14B). Focal-enhancing intra-axial masses or nonenhancing small white matter lesions may also be present. Calcifications are not typical. Differential diagnosis includes granulomatous CNS infections, metastatic disease, Wegener granulomatosis, and Langerhans cell histiocytosis.
primarily affects the leptomeninges, and abnormal leptomeningeal and dural enhancement can be seen with both CT and MR (Fig. 6.14A). Thickening and enhancement of the cranial nerves and the hypothalamic-pituitary axis are not uncommon (Fig. 6.14B). Focal-enhancing intra-axial masses or nonenhancing small white matter lesions may also be present. Calcifications are not typical. Differential diagnosis includes granulomatous CNS infections, metastatic disease, Wegener granulomatosis, and Langerhans cell histiocytosis.
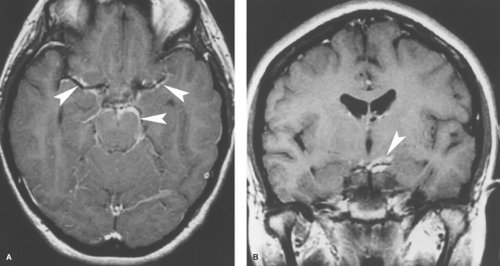 Figure 6.11. Coccidioidomycosis Meningitis. Contrast-enhanced transaxial (A) and coronal (B) T1WI reveal abnormal enhancement of the meninges in the basal cisterns (arrowheads). |
Parenchymal Infections
Pyogenic Cerebritis and Abscess
Bacterial infections of the brain may develop by direct extension following trauma, surgery, paranasal sinusitis, otomastoiditis, or dental infections. Hematogenously spread infections occur even more frequently, especially in patients with lung infections, endocarditis, or congenital heart disease. Anaerobic bacteria are the most common organisms overall. Infection with Staphylococcus aureus is common after surgery or trauma. Gram-negative rod, pneumococcal, streptococcal, listerial, nocardial, and actinomycotic infections also occur with some frequency. With infections resulting from hematogenous spread, the frontal and parietal lobes (middle cerebral artery distribution) are most commonly involved, with the abscess centered at the gray–white junction. The frontal lobes are most commonly affected with spread of sinus infections. The temporal lobes or cerebellum are involved in patients with spread from otomastoiditis.
Clinical symptoms in patients with pyogenic brain infections may be mild or severe. Headache is common. There may be varying degrees of lethargy, obtundation, nausea, vomiting, and fever. Fever is absent more than 50% of the time. Meningeal signs are present in only 30% of patients. Focal neurologic deficits, papilledema, nuchal rigidity, and seizures can develop rapidly over the course of a few days. This is in distinction to tumors, where these symptoms usually develop more slowly. There is often, but not invariably, an elevated white blood cell count. CSF studies are often nonspecific and may not be obtained because of the risk of herniation following lumbar puncture in the setting of a brain mass.
A solitary abscess is usually treated surgically. Often, stereotactic needle aspiration, followed by antibiotic therapy, is performed, especially if the abscess is in an eloquent area of the brain. If there is significant mass effect, or if the lesion is in a relatively “safe” area, a formal drainage or resection is performed. With early cerebritis, small or multiple abscesses, or if the patient is a poor surgical candidate, antibiotic therapy alone is used. Imaging studies should be performed frequently (perhaps weekly) to monitor the efficacy of treatment and to assess for complications such as herniation, infarction, and hydrocephalus.
The imaging appearance of cerebritis and brain abscesses evolves and corresponds with four pathologically described stages:
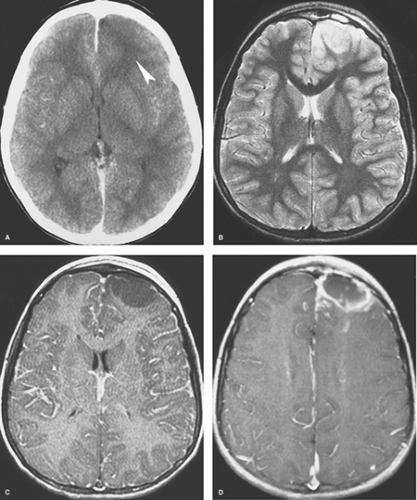
Early Cerebritis. Within the first few days of infection, the infected portion of the brain is swollen and edematous. Areas of early necrosis are filled with inflammatory polymorphonuclear leukocytes, lymphocytes, and plasma cells. Organisms are present in both the center and the periphery of the lesion, which has ill-defined margins. CT scans may be normal or show an area of low density (Fig. 6.15A). On MR, the lesion is hypointense or isointense on T1WI and hyperintense on T2WI and FLAIR images (Figs. 6.15B, C). There may be mild mass effect and patchy areas of enhancement within the lesion on both CT and MR. A ring of enhancement is not present at this stage, thus distinguishing it from the later three stages. Unfortunately, these imaging features are nonspecific and can be seen with neoplasms or infarcts. The clinical features are therefore most important in making the correct diagnosis. If the diagnosis can be made at this stage, nonsurgical treatment with antibiotics is often effective.
Late cerebritis occurs within 1 or 2 weeks of infection. Central necrosis progresses and begins to coalesce, with fewer organisms detected pathologically. There is vascular proliferation at the periphery of the lesion, with more inflammatory cells and early granulation tissue, which represent the brain’s effort to contain the infection. Not surprisingly, this corresponds to irregular contrast enhancement at the edges of the lesion on imaging studies (Fig. 6.16). Centrally, there is increased hypodensity on CT, hypointensity on T1WI, and hyperintensity on T2WI and FLAIR sequences on MR. DWI may show some increased signal intensity within the center of the lesion. Scans acquired after a delay following administration of contrast material may show some late central enhancement. There is worsening vasogenic edema present outside the enhancing rim and overall increased
mass effect. No discrete, T2-hypointense capsule is evident on MR as may be observed in some mature abscesses. This stage can also be treated effectively with antibiotic therapy, but distinguishing late cerebritis from an early abscess or tumor can be difficult and surgery is often performed.
mass effect. No discrete, T2-hypointense capsule is evident on MR as may be observed in some mature abscesses. This stage can also be treated effectively with antibiotic therapy, but distinguishing late cerebritis from an early abscess or tumor can be difficult and surgery is often performed.
Early Capsule. Within 2 weeks, the infection is walled off as a capsule of collagen and reticulin forms along the inflammatory, vascular margin of the infection. Macrophages, phagocytes, and neutrophils are also present in the capsule. The necrotic center contains very few organisms. Contrast-enhanced CT and MR scans show a well-defined, usually smooth and thin, rim of enhancement (Fig. 6.15D). The rim tends to be T2-hypointense. Central necrosis again results in hypodensity on CT and in T1-hypointensity and T2-hyperintensity on MR. Prominent surrounding vasogenic edema usually persists. There is reduced diffusion with hyperintensity centrally on DWI.
Late Capsule. In the late capsule stage, the rim of enhancement becomes even better defined and thicker, reflecting more complete collagen in the abscess wall (Fig. 6.17). Multiloculation is common. Prominent increased signal intensity present centrally on DWI is an extremely helpful imaging feature (Fig. 6.18C). The capsule often exhibits characteristic features on MR that are helpful diagnostically at this stage. On T1WI, the capsule is usually isointense or hyperintense to white matter, and on T2WI, it is usually hypointense to white matter (Figs. 6.18A, B). These signal characteristics suggest paramagnetic T1 and T2 shortening, similar to that seen during the evolution of hematomas (see Chapter 4). However, hemorrhage is not always found pathologically, and these paramagnetic effects, which may also reflect the presence of free radicals, produced by macrophages. Regardless of this, the MR appearance of the capsule is fairly specific for an abscess. The inner aspect of the enhancing capsule is often (about 50% of the time) thinner than the peripheral aspect (Figs. 6.17C, 6.18D). This reflects relatively decreased blood supply and fibroblast migration centrally compared with cortically. This thin medial rim predisposes to intraventricular rupture of the abscess and resulting ependymitis/ventriculitis (Fig. 6.17C). CT or MR scans reveal enhancement of the ependymal lining of the ventricles and altered density and signal intensity of the intraventricular CSF.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree