Central Nervous System Neoplasms and Tumor-Like Masses
Kelly K. Koeller
Although neoplasms of the central nervous system (CNS) are rare, these lesions garner exceptional interest because of the dramatic and sometimes catastrophic alteration they induce in the lives of affected patients. The overall annual incidence is approximately 20,000 new cases in the United States. Most (80% to 85%) occur in those older than 15 years, most commonly (70%) located in the supratentorial compartment. Metastatic lesions comprise about 30% of all CNS neoplasms in this age group. In contrast, tumors that arise in those younger than 15 years tend to be located in the posterior fossa (70%) and metastatic disease during the childhood age is rare. In terms of prevalence, CNS tumors are second only to leukemia during the childhood years.
While CNS neoplasms are classically categorized by neuropathologists according to their cell of origin, analysis of these lesions on imaging studies is perhaps best approached with regard to their anatomic location. This chapter will consider a broad spectrum of CNS tumors and tumor-like masses defined not only by their histologic composition but also grouped according to their common locations and consideration of the appropriate differential diagnosis.
Classification
The original classification scheme proposed by Bailey and Cushing in the 1920s serves as the foundation for the histological categorization of all brain tumors currently proposed by the World Health Organization (WHO). Basically, the WHO classification scheme recognizes seven major categories based on the cell of origin (Table 5.1). These include tumors of neuroepithelial cells, tumors of the nerve sheath (composed of Schwann cells and fibroblasts), tumors of the meninges (composed of meningothelial, mesenchymal, and melanocytic tumors), tumors of lymphoproliferative cells, tumors of germ cell origin, tumors of the sella, and metastatic disease. Each of these cells of origin gives rise to a particular tumor type. The tumors of neuroepithelial origin comprise the largest group and include tumors of glial origin (usually astrocytic tumors, oligodendroglial tumors, ependymal tumors, choroid plexus tumors), tumors of nonglial origin (e.g., ganglioglioma, central neurocytoma, and others), tumors of pineal origin (e.g., pineocytoma and pineoblastoma), and embryonal tumors (e.g., medulloblastoma and primitive neuroectodermal tumor).
The cell of origin directly impacts on tumor nomenclature. If the cellular composition is primarily astrocytes, then the tumor is called an astrocytoma. If the majority of the cells are oligodendroglial, then it is termed an oligodendroglioma, and so on. The brain itself is predominantly composed of neuroepithelial cells and hence the most common tumor type is derived from this cell line. Although the neuron is the most common cell type overall in the brain, mature neurons do not divide and thus cannot produce neoplastic growth. Therefore, most (40% to 50%) tumors of the brain itself are gliomas. Since they arise from the brain parenchyma itself, these tumors are virtually always “intra-axial” in location.
Since most nonglial tumors do not arise from neuroepithelial tissue in the brain parenchyma, they are overwhelmingly located outside of the brain proper, that is, along the coverings of the brain or within the ventricular system. Tumors arising from these “outside” locations are therefore referred to as “extra-axial” tumors. This concept of intra-axial and extra-axial is critical to the correct interpretation of cross-sectional CNS imaging studies. In general, the histologic composition of these tumors is directly linked with the location of the tumor.
Table 5.1 Intracranial Neoplasms and Their Cells of Origins | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
Clinical Presentation
The clinical presentation of a CNS neoplasm is almost always related to increased intracranial pressure, seizure activity, or a focal neurologic deficit.
Subfalcine Herniation. The falx is a very tough fibrous structure that is very resistant to any sort of displacement. When a mass is located in certain key locations or is of sufficient size, portions of the brain itself may be pushed across the midline or through dural openings. This displacement of the brain is called herniation and many types have been defined according to the brain structure that is most affected. Subfalcine (or cingulate) herniation is the most common type of herniation and occurs when the cingulate gyrus is displaced under the margin of the interhemispheric falx. Even if the cingulate gyrus is not displaced underneath the falx, significant general midline shift is considered to be present if the shift is 3 mm or greater.
Uncal and Central Herniation. The uncus represents the hooked extremity of the parahippocampal formation of the medial temporal lobe. Uncal herniation often compromises the many tracts running through the brainstem as well as cranial nerves, particularly the oculomotor (III) nerve causing an ipsilateral pupillary dilation (or “blown pupil”). On imaging studies, effacement of the ambient cistern and contralateral hydrocephalus are the hallmarks of uncal herniation. Central herniation is the result of either downward or upward displacement of the brainstem through the tentorial insura. It most commonly results from bilateral or midline supratentorial masses that cause complete obliteration of the cisternal spaces. Elongation of the brain stem in the anteroposterior axis and narrowing in the transverse axis is seen on axial images.
Hydrocephalus. The mass effect of an intracranial neoplasm may be sufficient by itself to produce increased intracranial pressure or hydrocephalus secondary to obstruction of the flow of cerebrospinal fluid (CSF) as it circulates through the ventricles and into the subarachnoid space. The increased pressure is associated with a classic clinical triad of headaches, nausea and vomiting, and papilledema (caused by partial obstruction of the venous outflow from the optic nerve). These features may occur at any time during the course of the brain tumor. In addition, altered mental status (particularly with bifrontal lobe tumors) or alterations in equilibrium
(commonly seen in cerebellar or eighth cranial nerve tumors) may be present. Intracranial neoplasms usually present with an indolent course marked by progressive headache and focal neurologic deficit, but may also present abruptly.
(commonly seen in cerebellar or eighth cranial nerve tumors) may be present. Intracranial neoplasms usually present with an indolent course marked by progressive headache and focal neurologic deficit, but may also present abruptly.
Approach to A Radiographic Abnormality
The detection of an intracranial abnormality on any imaging study should immediately provoke the following three questions:
Mass? By far the most important question to ask is: “Is it a mass?” It is important to consider that abnormal attenuation on computed tomography (CT) or signal intensity on magnetic resonance (MR) imaging does not necessarily equate to a “mass,” which, by definition, must have mass effect. In other words, it must displace normal brain structures. Many diseases may produce mass effect and therefore qualify as a “mass.” However, all tumors by their very nature should have mass effect. The mass effect from a very small tumor may be beyond the limits of detection on imaging studies but this is an infrequent event. Whenever a mass is encountered on an imaging study, a neoplasm is a prime consideration in the differential diagnosis.
Distinguishing an early infarct from a neoplasm may be problematic on CT but is straightforward on MR imaging studies, especially with diffusion-weighted imaging (DWI). If these studies are not available, a follow-up conventional imaging study (preferably with MR imaging) in 3 weeks’ time may be helpful. Virtually all infarcts will be smaller in size by 3 weeks after clinical presentation. If the lesion is the same size or larger at 3 weeks, a neoplasm should be favored. Also, as detailed in Chapter 4, a subacute infarct will often show signs of subtle hemorrhage. Obviously, as the treatment of tumor and infarct are dramatically different, the distinction between a tumor and an infarct is critical for appropriate clinical management of the patient.
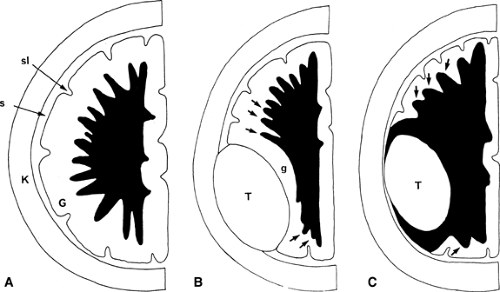
Intra-axial or Extra-axial? Once the presence of a mass has been determined, the next important question to ask is: “Is the mass intra-axial or extra-axial?” As presented earlier, an intra-axial mass is a mass that is of the brain itself (i.e., it arises from the brain parenchyma). An extra-axial mass refers to everything outside the brain (i.e., arachnoid, meninges, dural sinuses, skull, etc.). The ventricular system is also considered extra-axial. Determining the intra-axial or extra-axial location of a suspected neoplasm is crucial to formulating an appropriate differential diagnosis. Extra-axial lesions are characterized by “white matter buckling” or inward compression of the white matter (often with thinning of the fronds of the white matter) and maintenance of the gray matter–white matter interface (Fig. 5.1). In contradistinction, an intra-axial mass expands the white matter, thickens its fronds, and blurs the gray matter–white matter interface. However, white matter buckling is not foolproof in differentiating extra-axial from intra-axial lesions. Where extensive white matter edema is present, no buckling of the white matter may occur. Therefore, while the “white matter buckling” sign is helpful when present, its absence does not necessarily indicate that a lesion is intra-axial.
Tumor Margin? A third question often posed is: “Where’s the tumor margin?” The histologic examination of a typical brain tumor actually provides the answer. On microscopic analysis, every glioma and practically every intra-axial neoplasm lacks a capsule and therefore it is possible for neoplastic cells to migrate far from the apparent center of the tumor. The consequence is that there is no distinct margin for an intra-axial neoplasm. Therefore, knowing the margin is not possible on microscopy, it certainly is not possible by cross-sectional imaging. Treatment is typically directed to the entire region of abnormal hyperintensity on T2-weighted imaging (T2WI), not just the region described by enhancement on the T1-weighted postcontrast sequence. MRS is valuable in identifying areas of spread in regions with normal T2 signal.
Trying to make a histologic diagnosis from an MR or CT scan is fraught with hazard. However, it is possible to render an intelligent analysis of the mass, including assessment of signal intensities and enhancement characteristics of the mass, and to present an accurate differential diagnosis whenever one encounters a mass suspected of being a CNS tumor on an imaging study.
Imaging Protocol
Imaging evaluation of intracranial neoplasms is best conducted by MR, which is far superior to CT because of its multiplanar capability, increased contrast resolution, and lack of ionizing radiation. CT is superior to MR in the assessment of calcification, although the use of gradient-recalled echo (GRE) or susceptibility-weighted (SWI) sequences increases the sensitivity of the latter technique to calcification. CT is invaluable
for the evaluation of bony abnormalities, such as erosion of the skull base.
for the evaluation of bony abnormalities, such as erosion of the skull base.
MR. A basic MR evaluation of a patient suspected of having an intracranial neoplasm includes sagittal and axial T1-weighted sequences, an axial fluid-attenuated inversion recovery (FLAIR) sequence, an axial T2-weighted sequence, DWI, and postcontrast axial and coronal T1-weighted sequences. FLAIR imaging excels at demonstrating abnormal signal intensity involving the periventricular and peripheral regions but also is associated with more artifact in the posterior fossa. The unenhanced T1-weighted sequences allow distinction between inherent T1 shortening, such as in hemorrhage, and true contrast enhancement. For temporal lobe and midline lesions, the coronal plane usually provides the best delineation of the tumor. Postcontrast sagittal MR is often best for midline masses and may facilitate radiation therapy planning. Performance of all of these sequences can be easily completed within 45 minutes on 1.5 T MR units. In addition to improved contrast resolution and signal-to-noise resolution, 3 T MR scanning is being used with increased frequency and allows acquisition of volumetric sequences that may shorten the scanning. Perfusion imaging is increasingly used to assess for cerebral blood volume and other vascular parameters associated with brain tumors. Implementing gradient-moment nulling (flow-compensating) techniques facilitate evaluation of the posterior fossa by decreasing phase artifact generated by the dural sinuses. (Fig. 5.2). MR spectroscopy and metabolic imaging may also be valuable in many cases.
Appearance of Tumors
The cross-sectional imaging appearance of CNS tumors varies with their cellular composition and the presence or absence of hemorrhage and calcification. On CT, intra-axial neoplasms will typically appear as hypodense masses with a variable amount of surrounding white matter edema, the area of which roughly correlates with the biologic behavior of the tumor. On MR, the mass is usually dark on T1 (T1 prolongation) and bright on T2 (T2 prolongation) with variable surrounding vasogenic edema. The presence of calcification within the tumor usually produces marked hypointensity on T1WI and T2WI. Occasionally, because of the surface area of the crystals producing T1 shortening, calcification may appear bright on T1WI.
Nontumoral Hemorrhage. The appearance of intracranial parenchymal hemorrhage usually depends on the age of the blood. In hyperacute (less than 6 hours) hemorrhage, the predominant oxyhemoglobin will produce T1 and T2 prolongation (dark on T1 and bright on T2). When the hemorrhage has been present for 6 to 24 hours, the effect of deoxyhemoglobin predominates and the lesion has mild T1 prolongation (dark on T1WI) and moderate T2 shortening (darker on T2WI). After 3 to 4 days, methemoglobin begins to predominate, first being intracellular, producing T1 and T2 shortening (bright on T1WI and dark on T2WI) and then, as the red blood cells begin to lyse, becoming extracellular where the lesion has T1 shortening and T2 prolongation (bright on both T1WI and T2WI). In chronic hemorrhages (older than 10 to 14 days), hemosiderin appears, producing a rim of extreme T2 shortening. This peripheral markedly hypointense rim occurs because of migrating macrophages, which carry the hemosiderin to the periphery of the hemorrhage. On CT, acute hemorrhage (less than 1 week old) has increased attenuation (hyperdensity) compared to the normal brain tissue. By 1 to 3 weeks after the hemorrhage, the signal becomes isodense compared to the normal brain parenchyma. After 3 weeks, the focus of hemorrhage is hypodense to brain parenchyma, simulating the attenuation of CSF. This evolution of blood breakdown products is illustrated in detail in Chapter 4.
Tumoral Hemorrhage. The appearance of intratumoral hemorrhage reflects the heterogeneous nature of the tumor and is quite different compared to benign parenchymal hemorrhage. Intratumoral hemorrhage is often intermittent, producing a heterogeneous mixture of the various blood breakdown products just described. In addition, hemorrhage may occur in cystic or necrotic portions of the tumor, creating blood–blood or fluid–blood levels. Debris from the necrotic mass will also contribute to this heterogeneous mixture. Normal deoxyhemoglobin evolution is delayed such that it will persist for longer than the usual 3 to 4 days after hemorrhage. The typical hemosiderin ring does not form with intratumoral hemorrhage, probably due to interference with the migration of the macrophages by viable tumor at the margins. In cases where there is confusion as to the nature of an intracranial hemorrhage, the presence of a nonhemorrhagic mass adjacent to the hemorrhage, the persistence of T2 prolongation (most likely representing edema or tumor itself), and mass effect all suggest intratumoral hemorrhage instead of a simple parenchymal
hematoma. Gadolinium administration is often helpful in these cases, as benign hematomas should not have as significant enhancing rim as those from tumors.
hematoma. Gadolinium administration is often helpful in these cases, as benign hematomas should not have as significant enhancing rim as those from tumors.
Hemorrhagic Neoplasms. Because of their high vascularity, certain neoplasms are noted for their propensity to hemorrhage. Choriocarcinoma among primary tumors and metastases from melanoma, thyroid carcinoma, and renal carcinoma show this characteristic. In the setting of multiple hemorrhagic lesions within the brain, these tumors should be considered. Multiple cryptic arteriovenous malformations, either occurring de novo or secondary to radiation therapy, can have a similar appearance with the exception of surrounding vasogenic edema.
T1 Shortening. Besides hemorrhage, two other entities may produce focal T1 shortening on MR scans. Fat within lipomas or dermoids produces marked T1 shortening and intermediate signal on T2WI following the signal intensity of subcutaneous fat. The presence of chemical shift artifact on T2WI associated with such a lesion helps to confirm the presence of fat. Melanin, as seen in melanotic melanoma, also follows the same signal intensities as fat on T1WI and T2WI.
Hyperdense Neoplasms. Tumors of high cellular density, usually those with small cells such as lymphoma, pineoblastoma, neuroblastoma, or medulloblastoma, are usually hyperdense compared to brain tissue on CT. In addition, metastases from melanoma, lung carcinoma, colon carcinoma, and breast carcinoma may be hyperdense. On MR, these same tumors are typically hypointense on T2WI with the appearance presumably being related to a high nucleus-to-cytoplasm ratio of the tumor cells, which produces less free water and thus less T2 prolongation. On occasion, iso- or hyperintensity may be seen because of heterogeneity of the tumor matrix.
Enhancement. Contrast enhancement, whether from iodinated contrast agents used in CT or paramagnetic gadolinium agents used in MR, occurs based on one primary factor: breakdown of the blood–brain barrier. Unlike nonneural endothelium, the endothelium of the cerebral capillaries allows the passage of only small molecules through their tight junctions and narrow intercellular gaps. The macromolecules that make up contrast agents are too large to pass this barrier under normal circumstances. When the blood–brain barrier breaks down, contrast is able to leak across the barrier and abnormal contrast enhancement is seen. Many pathologic states, including intra-axial tumors (either primary or metastatic), inflammatory diseases, subacute infarcts, postoperative gliosis, and radiation necrosis among others, may be associated with this event. Some tumors, particularly low-grade neoplasms, will not show enhancement presumably because they form new capillaries that are quite similar to the native cerebral capillaries with the blood–brain barrier left intact. More biologically aggressive high-grade neoplasms tend to have fenestrated capillaries that allow the passage of contrast media and consequently show image enhancement. However, the fact that a lesion enhances means that there is a breakdown of the blood–brain barrier, and the presence or absence of enhancement cannot be used to categorically state that a lesion is low-grade or high-grade (Fig. 5.3). In addition, some specialized areas of the brain, such as the choroid plexus, pituitary and pineal glands, tuber cinereum, and area postrema, have no blood–brain barrier and will normally enhance after administration of a contrast agent.
The Postoperative Patient
In the evaluation of a postoperative brain tumor patient, timing is of the essence. It is recognized that postoperative granulation tissue develops within 72 hours following surgery and enhances after administration of contrast. Once formed, this tissue may persist for weeks to months. Since most malignant brain tumors have at least some enhancement, the presence of enhancement on a study performed during this 72-hour window is generally regarded as being related to residual tumor rather than granulation tissue.
Safety. Postoperative neurosurgical patients are often not ideal candidates for scanning in an MR unit and proper monitoring of vital signs to assure their safety is of paramount importance. If the appropriate monitoring and life-support equipment (e.g., shielded pulse oximeter and oxygen) and personnel are not available to safely perform an MR study, a monitored contrast-enhanced CT should be substituted.
The Follow-Up Scan
Many malignant tumors will be treated by a combination of chemotherapy and radiation therapy following surgical debulking. Typical radiation doses are in the range of 5000 to
5400 rad, most often delivered in fractionated doses (about 180 rad each visit) over several weeks time. As a consequence, radiation injury in the white matter occurs in two forms: diffuse white matter injury and radiation necrosis.
5400 rad, most often delivered in fractionated doses (about 180 rad each visit) over several weeks time. As a consequence, radiation injury in the white matter occurs in two forms: diffuse white matter injury and radiation necrosis.
Despite the widespread involvement seen in diffuse white matter injury, most patients do not have any neurologic deficits. A “geographic distribution” of white matter hyperintensity on T2WI conforming to the selected radiation ports is typical for diffuse white matter radiation injury and should not be misinterpreted as vasogenic edema from the tumor. Virtually all patients following whole-brain or large-volume radiation will demonstrate this pattern of involvement 6 months or more after the therapy is completed. Affected areas do not enhance on postcontrast imaging. Distinction from a brain tumor is seldom a problem in the setting of diffuse white matter injury.
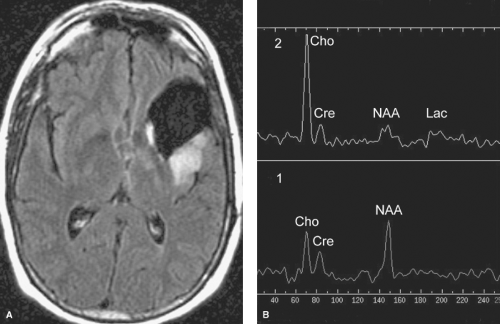
In contrast, the much less commonly seen radiation necrosis is virtually indistinguishable from that of recurrent tumor on CT or conventional MR images as both usually have mass effect and enhancement. Clinically, patients with radiation necrosis often present with focal neurologic deficits. However, MRS, DWI, perfusion imaging, and metabolic imaging (PET or SPECT-thallium studies) are useful in making this distinction. While radiation necrosis may have an elevated lactate peak on MRS secondary to necrosis, the elevated choline peak and depressed N-acetyl aspartate (NAA) peaks expected with tumors are usually absent (Fig. 5.4). Necrotic areas typically show restricted water diffusion (hyperintensity on DWI and corresponding hypointensity on apparent diffusion coefficient (ADC) map images). In contrast, the overwhelmingly majority of brain tumors do not cause restricted water diffusion and will not be hyperintense on DWI. Similarly, perfusion imaging may distinguish between radiation necrosis and tumor, as some tumors (particularly those of higher grade) show increased perfusion while there is absence of increased perfusion in radiation necrosis. With metabolic imaging, radiation necrosis shows normal to decreased metabolic activity while recurrent tumor is usually increased, especially if the original tumor was high-grade (e.g., WHO grade III or IV; see “Astrocytomas” section). Distinguishing between recurrent tumor and radiation necrosis using metabolic imaging is less accurate if the original brain tumor was a WHO grade I or II tumor.
Specific Neoplasms
It is difficult, in many circumstances, to suggest a specific histologic diagnosis based on the imaging characteristics alone. However, taking into account other factors such as the location of these tumors (intra-axial, extra-axial, intraventricular, sellar region, pineal region) and clinical information (age, gender, endocrinologic data, etc.), the differential diagnosis can be limited to just a few possibilities and sometimes a single most likely entity. Some intracranial tumors have a definite predilection for one gender (see Table 5.2).
Intra-axial Tumors: Glial
Gliomas, derived from glial cells, account for 40% to 50% of all primary CNS neoplasms. Most of these tumors are histologically regarded as astrocytomas and oligodendrogliomas.
Table 5.2 Tumor Predominance By Gender | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Astrocytomas. Account for 70% of all gliomas. They are graded according to five histologic features: cellularity, mitotic activity, pleomorphism, necrosis, and endothelial proliferation. Four grades are currently recognized. The grade I tumors are generally well-circumscribed on imaging and gross pathologic inspection. The pilocytic astrocytoma and subependymal giant cell astrocytoma are the prototypical examples of this tumor type. A diffuse astrocytoma, lacking the well-circumscribed morphology seen in the grade I tumors but with a low degree of cellularity, mitotic activity, and pleomorphism, characterizes the grade II tumors. Specifically, these tumors lack necrosis and endothelial proliferation. Grade III tumors, termed anaplastic astrocytoma, demonstrate increased amounts of cellularity, mitoses, and pleomorphism on histology. The most malignant form (WHO grade IV) of an astrocytoma, the glioblastoma multiforme (GBM), has marked amounts of cellularity, mitotic activity, and pleomorphism (as the name “multiforme” would imply). However, in distinction to the other types described, extensive necrosis and endothelial proliferation are prominent features of this tumor. All glial tumors lack a capsule and therefore have at least the potential for spread throughout the CNS. Still, the lower-grade (WHO grades I and II) astrocytomas and the higher-grade (WHO grades III and IV) astrocytomas are associated with certain distinct clinical and morphologic features that emphasize the differences in prognosis for patients with these tumors.
Lower-grade tumors. Since the lower-grade WHO grades I and II astrocytomas are usually so slow-growing and exhibit such nonaggressive behavior, patients with these tumors often do well with surgical resection alone. Prognosis for these tumors is measured in terms of years. These astrocytomas tend to occur in younger patients, usually children and adults 20 to 40 years old. They are usually well-demarcated tumors without necrosis or neovascularity, rarely hemorrhage, and are often cystic. They show calcification in 20% of cases and rarely have surrounding edema. On CT, they are hypodense with little or no enhancement. On MR, compared to gray matter, they are hypointense on T1WI, hyperintense on T2WI, and show minimal enhancement (Fig. 5.5).
Higher-grade tumors. In contrast to the lower-grade astrocytomas, the higher-grade astrocytomas tend to occur in patients older than 40 years. These tumors are poorly delineated microscopically, although they may appear well-circumscribed grossly. Necrosis, hemorrhage, and neovascularity are common, particularly in the GBM. Surrounding white matter edema is very common (Table 5.3). On CT, they are typically heterogeneous. On MR, they are iso- to hypointense compared to gray matter on T1WI and hyperintense on T2WI. A ring-like pattern on postcontrast imaging may be seen (Fig. 5.6).
Pathology. Astrocytomas may demonstrate a paradox in their gross appearances. The well-differentiated low-grade astrocytomas are frequently ill-defined as they insinuate themselves through the neurons and other supporting cells that make up the “scaffold” of the brain parenchyma, whereas the highly-malignant GBM is macroscopically better circumscribed. In truth, all astrocytomas are poorly circumscribed upon microscopic examination.
Spread. Gliomas spread from their native site by one of four ways. They may spread via the white matter tracts, such as the corona radiata, corticospinal tracts, corpus callosum, and hippocampal commissures. They may spread by way of either natural passages such as the perivascular (Virchow-Robin) spaces or along the subpial or subependymal surfaces. Finally, tumors may also rarely spread across the meninges.
Glioblastoma multiforme (GBM), the most malignant form of an astrocytoma, is also the most common type of glioma. The peak age of incidence is 45 to 55 years, with males slightly more commonly affected. The deep white matter of the frontal lobe, the largest lobe in the brain, is the most common location followed by the temporal lobe and the basal ganglia.
Imaging. The cross-sectional imaging appearance reflects the pattern of necrosis, hemorrhage, and neovascularity seen microscopically. The classic appearance on either CT or MR is an expansile mass with central necrosis, ring-enhancement, and a large surrounding region of vasogenic edema. On noncontrast CT, the tumor is typically heterogeneous and lobulated with marked surrounding white matter edema. Calcification may be seen occasionally. Necrosis and hemorrhage are common. The most common hemorrhagic neoplasms in the brain are GBM, metastasis, and oligodendroglioma (Table 5.4). On MR, the tumor nidus commonly shows T1 and T2 prolongation (dark on T1WI and bright on T2WI) compared to gray matter (Fig. 5.6). Because of cellular debris from the necrosis, the signal intensity of these cyst-like areas is usually slightly different from that of CSF. Reflective of the endothelial proliferation seen histologically, the tumor tends to be highly vascular. Multiple markedly hypointense holes representing flow voids may occasionally be seen. Perfusion imaging typically demonstrates regions of increased relative cerebral blood volume (rCBV) in GBM, which correlate with areas of increased biologic behavior (higher histopathologic grade) and may direct potential sites for stereotactic biopsy of suspected tumors.
Ring enhancement. On contrast-enhanced CT and MR, more than 90% of all GBMs will show at least some enhancement, usually in an irregular, occasionally nodular, ring-like pattern. Many other lesions can present with as ring-enhancing masses. A convenient way to remember these entities is by the mnemonic “MAGIC-DR” (Table 5.5). The first three entities (metastasis, abscess, and glioma) are by far the most common causes of this appearance and are listed in order of frequency. The “irregular ring” enhancement of a neoplasm is often distinct from the typical “smooth ring” seen in cerebral abscesses (compare Fig. 5.6 with Fig. 6.3A). Furthermore, an abscess rim typically is hyperintense on T1WI and hypointense on T2WI features not commonly seen in tumors.
Butterfly Glioma. GBM is one of two entities (CNS lymphoma is the other) that commonly may have bihemispheric spread through the corpus callosum with involvement of both frontal lobes. Because the imaging appearance somewhat resembles the wings of a butterfly, such masses are commonly referred to as “butterfly gliomas.” It is important to recognize that abnormal signal intensity within the corpus callosum is secondary to the disease process itself and does not simply represent vasogenic edema. This is because the callosal fibers and projection fibers of the internal capsule are packed so tightly together that edema fluid cannot be conducted through them. Therefore, if a neoplasm is suspected, any T2 hyperintensity seen in the corpus callosum or internal capsule must be considered secondary to neoplastic spread and not from vasogenic edema. Other diseases, ranging from infection to
demyelinating disease, may also involve the corpus callosum and produce the butterfly glioma appearance.
demyelinating disease, may also involve the corpus callosum and produce the butterfly glioma appearance.
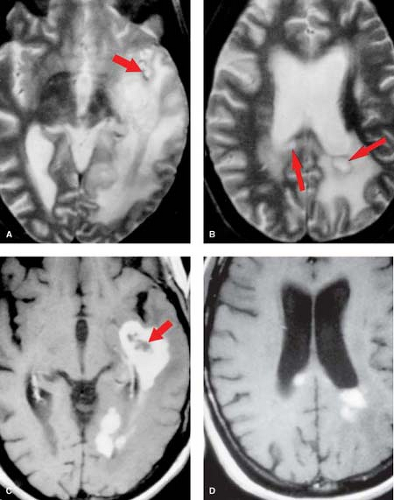
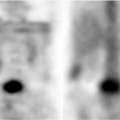
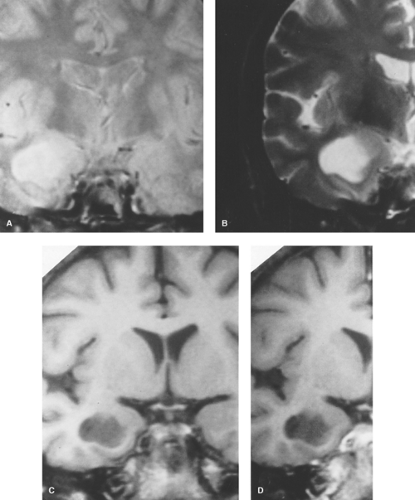 Figure 5.5. Coronal T2WI (A and B) show hyperintense well-defined temporal lobe mass. Precontrast (C) and postcontrast (D) T1WI demonstrate a hypointense mass without enhancement. |
Table 5.3 Intra-Axial Lesions with Marked Surrounding Edema | |
|---|---|
|
Treatment and prognosis. Surgical resection, chemotherapy, and radiation therapy are standard treatments for patients with such tumors. Reduction in size in association with some symptomatic improvement is typically noted. Treated lesions are often extensively necrotic and calcified. Up to 50% of patients who receive chemoradiotherapy for a GBM show increased size and prominence of abnormal signal intensity and enhancement in the area of the original tumor on follow-up MR scans within the first 3 to 6 months after surgery. While this may be related to true disease progression, about half of these cases
actually represent radiographic “pseudoprogression,” marked by radiologic deterioration without true disease progression. The etiology of this phenomenon is not well-understood but its recognition is important because patients with this condition do not require changes in therapy or additional surgery and average survival time in these patients is actually increased compared to those with true disease progression. The average overall survival time for all GBM patients is about 15 months. Therefore, the prognosis for these patients is much worse compared to those with the lower-grade astrocytomas. New treatment modalities, such as gamma-knife surgery and more advanced chemotherapy protocols, particularly those using temozolomide, are under constant evaluation in the hopes of improving the poor outlook for affected patients.
actually represent radiographic “pseudoprogression,” marked by radiologic deterioration without true disease progression. The etiology of this phenomenon is not well-understood but its recognition is important because patients with this condition do not require changes in therapy or additional surgery and average survival time in these patients is actually increased compared to those with true disease progression. The average overall survival time for all GBM patients is about 15 months. Therefore, the prognosis for these patients is much worse compared to those with the lower-grade astrocytomas. New treatment modalities, such as gamma-knife surgery and more advanced chemotherapy protocols, particularly those using temozolomide, are under constant evaluation in the hopes of improving the poor outlook for affected patients.
Table 5.4 Hemorrhagic Tumors | |
|---|---|
|
Table 5.5 Ring-Enhancing Lesions (“Magic Dr”) | |
|---|---|
|
Lower-Grade Astrocytomas are characterized by slow growth and associated with a longer clinical course. Patients often have productive lives for many years following diagnosis. These tumors account for 20% to 30% of all gliomas. Males are slightly more frequently affected and the peak incidence is between 30 and 40 years of age. In children, these tend to occur along the optic pathways, hypothalamus, and near/in the third ventricle. In adults, the lesions are usually located in the cerebral hemispheres.
Pathology. Lower-grade astrocytomas are pathologically divided into the fibrillary astrocytoma (WHO grade II), the pilocytic astrocytoma (WHO grade I), and the subependymal giant cell astrocytoma (WHO grade I). (The pilocytic astrocytoma will be discussed separately in the “Posterior Fossa Neoplasms” section.) Gemistocytic astrocytoma (WHO grade II) and protoplasmic astrocytoma (WHO grade II) are rare variants of the fibrillary form. The pleomorphic xanthoastrocytoma (WHO grade II) is a distinct clinicopathologic entity, primarily seen in adolescents and young adults, and is characterized by a heterogeneous mass with a soft tissue component located peripherally along the meningocerebral interface. It is believed that approximately 10% of all lower-grade astrocytomas will degenerate into a more aggressive WHO grade III or IV tumor.
Imaging. On CT and MR, these lesions generally have variable amounts of surrounding vasogenic edema and variable enhancement. Less than 50% will show enhancement in any portion of the mass. They may not be apparent on either noncontrast or contrast-enhanced CT and rarely may not even have any abnormal T2 hyperintensity. Calcification (25% of cases) and hemorrhage may be present but necrosis does not occur. The tumors are usually poorly marginated with mild mass effect (Fig. 5.5). The variable appearance may occasionally make distinction from an acute infarct difficult. In such circumstances, further evaluation with advanced MR techniques (e.g., DWI) may aid in establishing the correct diagnosis.
Treatment and prognosis. Surgical resection is the primary therapeutic approach, with chemotherapy and radiation therapy used according to the histologic grade and by experimental protocol. Recurrence is much less common compared to the GBM and patients with these tumors enjoy a corresponding better outlook with a mean survival time of 6 to 8 years.
Gliomatosis Cerebri is a rare neuroepithelial neoplasm of unknown origin that is the result of widespread infiltration of neoplastic cells (probably astrocytes) in varying degrees of differentiation. By definition, at least three lobes of the brain are involved. Despite the diffuse involvement of the brain seen pathologically and on imaging studies, the clinical symptoms are often mild. Peak incidence is between 40 and 50 years of age but it may occur at any time of life. Frequently, the lesion appears to smolder for weeks to years before erupting into a full-blown GBM or anaplastic astrocytoma. Radiotherapy may temporarily improve the radiologic appearance and improve clinical symptoms. The long-term prognosis is poor with a less than 30% 3-year survival rate.
Imaging. There are two basic imaging appearances of gliomatosis cerebri, either as diffuse involvement of the cerebral white matter without a mass or one with a discrete mass. In the former, the CT appearance of gliomatosis cerebri is almost always normal, as the lesions are isodense to normal brain parenchyma and do not enhance. MR plays an important role in establishing this diagnosis, particularly in assessing the diffuse involvement of the brain. Involved regions are characterized by diffuse T1 and T2 prolongation throughout the white matter and gray matter, particularly the centrum semiovale, hypothalamus, basal ganglia, and thalamus. The relative lack of mass effect in this form of the disease is striking. Distinction between the gray matter and white matter is often lost. Enhancement is typically absent unless a distinct mass is present. This appearance may be quite similar to that seen in progressive multifocal leukoencephalopathy occurring in immunocompromised patients.
The second imaging appearance includes all of the features described above with the addition of a focal mass, which in most circumstances represents a WHO grade III or higher lesion. Evaluation with MRS is helpful in identifying the more biologically aggressive regions and directing potential sites for surgical biopsy.
Oligodendroglioma accounts for 5% to 18% of all gliomas (about 4% of all intracranial neoplasms). It is more common in adults with a peak age of 30 to 50 years. Children are affected in about 6% of cases. The tumor is supratentorial in 85% of cases and most commonly (50% to 65%) located in the frontal lobe. The tumor usually grows slowly and, on microscopy, shows calcification in 100%, with hemorrhage and cysts occurring in about 20% of cases. Hematogenous or subarachnoid spread is uncommon. Accordingly, the tumor warrants a WHO grade II designation. However, the postoperative survival rates for patients with this tumor are quite variable and disappointing, with 38% to 75% 5-year survival rate and 20% to 60% 10-year survival rate.
Frequently, the tumor is combined with elements of astrocytes and is accordingly labeled as a “mixed glioma” (e.g., oligoastrocytoma). Many of these tumors show 1p–19q deletion on genotyping, which is strongly affiliated with the classic histopathologic findings in well-differentiated oligodendrogliomas and is associated with longer survival times.
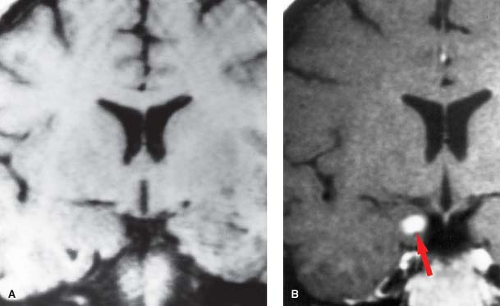
Imaging. Oligodendroglioma is most commonly located in the frontal lobes and often extends to the cortex where it may erode the calvarium. On CT, calcification is reported in up to 91% of cases compared to about 25% of astrocytomas (Fig. 5.7). However, since the astrocytoma is so much more common compared to the oligodendroglioma, a calcified tumor in the brain is more likely to be an astrocytoma rather than an oligodendroglioma (Table 5.6). On MR, it is usually hypointense on T1WI and hyperintense on T2WI compared to gray matter. Surrounding vasogenic edema is uncommon. Following contrast administration, about 66% show some enhancement although the degree of enhancement is variable. The appearance in an adult of a heterogeneous calcified mass within the periphery of a frontal lobe with calvarial erosion and relative absence of edema should suggest the diagnosis of an oligodendroglioma. Variant forms include oligoastrocytoma and anaplastic oligodendroglioma. While the imaging appearance of the former is practically indistinguishable from oligodendroglioma, the latter tumor may mimic the appearance of a GBM.
Intra-axial Tumors: Nonglial and Mixed Glial
Primary CNS Lymphoma. The incidence and demographics of primary CNS lymphoma have changed dramatically as a consequence of the increasing population of immunocompromised patients, particularly those with acquired immunodeficiency
syndrome (AIDS). Once considered extremely rare as a primary neoplasm, this tumor (almost always a B-cell Non-Hodgkin lymphoma) is now the fourth most common primary CNS neoplasm (following GBM, meningioma, and low-grade astrocytoma). There are some predictions that it will become the most common primary CNS neoplasm. Confusion, lethargy, and memory loss are common clinical symptoms. Interestingly, the tumor is exquisitely sensitive to steroid therapy and radiotherapy initially, only to rebound with a vengeance. This has led some to coin the phrase “ghost tumor” to describe this response. Consequently, it has been advocated to withhold steroid
therapy prior to neurosurgical biopsy as the steroid treatment may interfere with accurate histologic interpretation. Recent therapeutic advances have led to some multiyear survivals.
syndrome (AIDS). Once considered extremely rare as a primary neoplasm, this tumor (almost always a B-cell Non-Hodgkin lymphoma) is now the fourth most common primary CNS neoplasm (following GBM, meningioma, and low-grade astrocytoma). There are some predictions that it will become the most common primary CNS neoplasm. Confusion, lethargy, and memory loss are common clinical symptoms. Interestingly, the tumor is exquisitely sensitive to steroid therapy and radiotherapy initially, only to rebound with a vengeance. This has led some to coin the phrase “ghost tumor” to describe this response. Consequently, it has been advocated to withhold steroid
therapy prior to neurosurgical biopsy as the steroid treatment may interfere with accurate histologic interpretation. Recent therapeutic advances have led to some multiyear survivals.
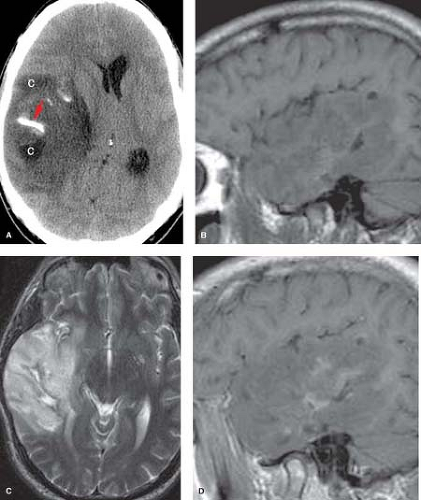 Figure 5.7. Oligodendroglioma. A. Noncontrast axial CT shows right temporal lobe mass with calcification (arrow) and cyst-like areas (C). B. Precontrast sagittal T1WI reveals the large ill-defined mass with predominant hypointensity. C. Axial T2WI demonstrates diffuse hyperintensity with exophytic extension beyond normal cortical margin and minimal surrounding vasogenic edema. D. Postcontrast sagittal T1WI shows patchy enhancement. (Published previously as part of the Neuroradiology Categorical Course Syllabus of the 2007 American Roentgen Ray Society 107th Annual Meeting.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|