Sinovenous thrombosis in children is rare, and the symptoms and signs are nonspecific especially in the neonatal population. MR imaging seems to be the most sensitive for accurate diagnosis of dural sinus thrombosis. General medical and neurologic supportive care is the mainstay of treatment. However, more active medical treatment such as anticoagulation, as well as an aggressive form of treatment such as retrograde transvenous fibrinolytic therapy, in children whose condition declines despite adequate anticoagulation therapy can be justified.
Cerebral venous thrombosis usually involves the cerebral venous sinuses, such as superior sagittal sinus and transverse sinus, but may involve deep venous system or cerebral cortical veins with isolated form or as part of a diffuse thrombotic process. Sinovenous thrombosis (SVT) in children is rare, and the symptoms and signs are nonspecific especially in the neonatal population. Thus, the diagnosis of SVT could be delayed or easily misdiagnosed. However, recent growing clinical awareness and improved noninvasive imaging techniques such as MR imaging have allowed us to diagnose the SVT in children earlier and more promptly.
Epidemiology
It is estimated that SVT constitutes approximately 20% of ischemic cerebral vascular disease in children. The incidence of SVT was 0.67 cases per 100,000 children per year between term birth to 18 years old. In that group, 43% of children who had SVT were neonates, and 54% were less then 1 year old, showing 97% of children who had SVT were developed within a year after birth . Carvalho and colleagues described a group of 31 children who had SVT; the median age of this group was 14 days, and 61% of patients were neonates. The incidence of the SVT is likely to increase with certain medical conditions, including prematurity, leukemia, and heart disease. Improvements in neuroimaging techniques such as MR venography and CT venography also contribute to a rise in the incidence of SVT .
Pathophysiology
Thrombosis of the venous system can occur due to venous stasis, prothrombotic status, involvement of the vessel wall, and endovascular deposition of embolic materials. A slow blood flow also favors the formation and propagation of thrombus in the venous system.
Venous infarction can occur when the pressure in the venous system rises above the arterial perfusion pressure. Thus, possible mechanism of venous infarction in SVT could be explained as follows. The obstruction of venous outflow due to the venous thrombosis raises the venous pressure in the area of the brain that needs to be drained through the occluded vein or sinus (venous congestion). The local venous congestion leads to extravasations of fluid and blood into the brain parenchyma (hemorrhagic infarction). Hydrocephalus can be developed in the cases of major sinus thrombosis and occlusion probably due to impairment in the absorption of cerebrospinal fluid through the arachnoid granulations .
Pathophysiology
Thrombosis of the venous system can occur due to venous stasis, prothrombotic status, involvement of the vessel wall, and endovascular deposition of embolic materials. A slow blood flow also favors the formation and propagation of thrombus in the venous system.
Venous infarction can occur when the pressure in the venous system rises above the arterial perfusion pressure. Thus, possible mechanism of venous infarction in SVT could be explained as follows. The obstruction of venous outflow due to the venous thrombosis raises the venous pressure in the area of the brain that needs to be drained through the occluded vein or sinus (venous congestion). The local venous congestion leads to extravasations of fluid and blood into the brain parenchyma (hemorrhagic infarction). Hydrocephalus can be developed in the cases of major sinus thrombosis and occlusion probably due to impairment in the absorption of cerebrospinal fluid through the arachnoid granulations .
Risk factors
SVT may be associated with various local or systemic conditions, such as prematurity, leukemia, and heart disease. Children who have SVT frequently have risk factors that tend to be related to the patient’s age. For example, only less than 5% of children who had SVT showed no predisposing risk factor .
Neonates are especially susceptible to SVT. Acute systemic illnesses were present in 84% of neonates in the Canadian stroke registry. The most frequently associated medical conditions include pre- or perinatal complications, such as asphyxia at birth, prolonged rupture of membranes, gestational diabetes, and maternal infections. Other neonatal conditions associated with SVT are dehydration, bacterial sepsis, meningitis, and various prothrombotic disorders. Although prothrombotic disorders were described in 15% to 20% of SVT in neonates , the contribution of those conditions to SVT is not clear. Fitzgerald and colleagues demonstrated gestational/delivery factors were present in 82%, and other comorbid conditions, such as dehydration, sepsis, and cardiac defects, were present in 62% in a group of 42 children who had neonatal SVT.
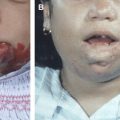
In infants who have SVT, head and neck disorders were more common. Most head and neck disorders were infections related to otitis media and mastoiditis . Iron-deficiency anemia is frequently associated with SVT in older children. Sébire and colleagues reported that 62% of children who had SVT, aged 3 weeks to 13 years, had anemia and/or microcytosis including iron deficiency, hemolytic, sickle cell, and β-thalassaemia. In older children who have SVT, chronic systemic diseases are present in approximately 40% to 60% of cases. These include cardiac diseases, connective tissue disorders, inflammatory bowel diseases, hematologic disorders, and nephrotic syndrome . Children who have chronic illnesses are prone to develop sinovenous thrombosis, probably secondary to an acquired hypercoagulable state. Acute illnesses like sepsis or dehydration are also important in the older age group of children. Recent head trauma and recent surgery that resulted in sinus damage represent approximately 13% of SVT cases .
Prothrombotic disorders, whether acquired or inherited, are important in the pathogenesis of SVT in children. Patients who have a malignant tumor such as non-Hodgkin lymphoma, leukemia, and neuroblastoma, may have various coagulation abnormalities leading to a prothrombotic state. Chemotherapy may also be associated with SVT.
Tests for prothrombotic disorders have revealed abnormalities in approximately 30% to 65% of children, including the presence of anticardiolipin antibodies; lupus anticoagulant; factor V Leiden; or the prothrombine-gene mutations; and decreased level of Protein C, antithrombin, Protein S, fibrinogen, or plasminogen . Among them, anticardiolipin antibody is the most frequently acquired abnormality, and the presence of factor V Leiden is the most frequent genetic abnormality . The acquired prothrombotic states seem to be more frequent then the inherited. The acquired deficiencies can include decreased level of antithrombin, protein C, protein S, anticardiolipin antibody, or lupus anticoagulant. They may be caused by an acquired disorder, such as infection, liver disease, nephrotic syndrome, or disseminated intravascular coagulation . The impact of congenital prothrombotic disorders such as factor V Leiden mutation or prothrombin mutation 20210 in children who have SVT is less clear . At all ages, there is a combination of a prothrombotic disorder and an acute illness in patients who have SVT.
Malformations of the sinuses and intracranial arteriovenous shunts, such as vein of Galen aneurysmal malformation (VGAM) or dural arteriovenous shunts are also known to induce or be associated with SVT.
Arteriovenous shunts and malformations of the dural sinuses may be seen before or at the time of the thrombotic episode. These vascular disorders can be combined with a coagulation disorder. A recent patient who had VGAM in our series who was completely treated by endovascular technique developed venous thrombosis episode. This episode is “unrelated” to the previously treated arteriovenous shunt, but a factor V Leiden deficit was confirmed after subsequent investigations. Another child presented with focal seizures related to parietal lobe cortical venous thrombosis few years after complete exclusion of his VGAM. Thus, venous thrombosis could be a responsible mechanism for various clinical episodes in the natural history of VGAM, cerebral arteriovenous malformation, dural arteriovenous shunt, dural sinus malformation, and even maxillo-facial arteriovenous malformation.
Imaging
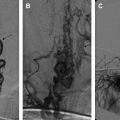
Widespread availability of power doppler ultrasound, contrast enhanced CT, and MR imaging, has resulted in increased and earlier diagnosis of SVT in children. The conventional angiography is rarely performed to make such diagnosis. The degree of clinical suspicion, the quality of the examination, and the radiologic interpretation can impact the accuracy of these imaging studies.
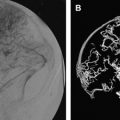
MR imaging and MR venogaphy ( Fig. 1 ) seems to be the most sensitive for accurate diagnosis of dural sinus thrombosis; however, it could be nearly equal for other modalities such as CT and CT venography ( Fig. 2 ) as well as ultrasound examinations in centers of imaging excellence. Detection of cortical vein thrombosis without dural sinus thrombosis either requires detailed MR imaging examination with special sequences or careful selective cerebral angiography. Conventional cerebral angiography can document the extension of the lesion and the quality of the venous collateral pathways. Because of their noninvasive nature, the MR and CT venography can be used as excellent follow-up imaging modalities.