Cervical arterial dissections and dissecting aneurysms are relatively rare pathologies, but can be associated with significant morbidity from ischemic complications. We review the challenges in diagnosing cervical arterial dissections, their unique clinical presentations and imaging characteristics. Although the majority of cervical dissections heal spontaneously with medical management, we discuss the specific indications for surgical or endovascular treatment to prevent thromboembolic complications. Furthermore, we provide a detailed technical review on endovascular stent reconstruction, the primary interventional option for symptomatic cervical dissections and dissecting aneurysms refractory to medical management.
Pathogenesis and epidemiology
Cervical dissections result from intimal injury, laceration of the arterial wall or spontaneous hemorrhage of the vasa vasorum causing a subintimal or intramural hematoma. Although dissections may remain asymptomatic, mass effect from the subintimal false vascular channel or intramural hematoma can narrow the true vessel lumen ranging from minimal compromise to turbulent and impaired flow across the stenotic segment. Severe dissections may ultimately progress to complete vessel occlusion. If antegrade flow persists in the false lumen, it is termed a “double-barrel” dissection with parallel patent lumens converging distally into the true lumen. If an intramural hematoma expands peripherally into the tunica media and subadventitial space, dissecting aneurysms or pseudoaneurysms may develop with inflow/outflow zones communicating by way of the true lumen.
The incidence of spontaneous cervical (carotid/vertebral) dissections is low with approximately three to five cases per 100,000, but they contribute to as many as 10% to 20% of thromboembolic strokes in young and middle-age patients. Although there is a peak incidence in the fifth decade of life with comparatively younger female presentations, there is no definite sex predilection.
Spontaneous etiologies are thought to be related to an inherent arteriopathy caused by genetic factors and connective tissue disorders such as Ehlers-Danlos syndrome type IV, Marfan syndrome, autosomal dominant polycystic kidney disease, osteogenesis imperfecta type I, and fibromuscular dysplasia (responsible for approximately 15% of spontaneous dissections). In addition, spontaneous dissections are associated with intracranial aneurysms, coarctation of the aorta, bicuspid aortic valve, a widened aortic root, arterial redundancy and distensibility. Using skin biopsy samples, Brandt and colleagues demonstrated structural abnormalities in the extracellular matrix of subjects with spontaneous dissections, analogous to the collagen and elastic fiber abnormalities in patients with Ehlers-Danlos and Marfan syndromes respectively, suggesting underlying collagen vascular disease.
Traumatic and iatrogenic dissections are predominantly due to blunt or penetrating injuries, chiropractic manipulation, or catheter angiography. The extracranial cervical arteries may be more susceptible to traumatic injury due to their mobility within the neck soft tissues along the cervical spine and tethering at the skull base; hence, the potential for direct injury from the adjacent bony structures.
Clinical presentation
Patients with cervical dissections present after minor or major inciting events resulting in neck hyperextension or rotation, often with sudden transitional or decelerating movements. Although dissections can occur following major head and neck trauma associated with motor vehicle accidents, many patients have a history of more subtle trauma such as practicing yoga, painting a ceiling, coughing, vomiting, sneezing, recent catheter angiography, anesthesia or resuscitation. In particular, chiropractic manipulation is notorious for causing carotid, and more frequently, vertebral artery dissections with 1 in 20,000 manipulations resulting in stroke.
Internal carotid artery dissections may present with the classic triad of ipsilateral headache or neck pain, partial Horner’s syndrome, and ischemic symptoms. However, lower cranial nerve palsies (particularly the hypoglossal nerve), impairment of taste (facial and glossopharyngeal nerve palsies), pulsatile tinnitus (or auscultation of a bruit), and cerebral and retinal transient ischemic attacks or infarcts can also be present at the time of initial presentation.
Nearly two thirds of patients with carotid dissections present with neck pain or headache usually described as a gradual onset of constant dull or aching pain, but acute presentations with severe throbbing, sharp pain, or even thunderclap headache may be seen. Anterolateral cervical pain associated with frontal-temporal headache or orbital/facial pain is usually the initial manifestation before ischemic symptoms. Similarly, transient ischemic attacks or transient monocular blindness can precede frank cerebral and retinal infarcts, with a relatively short time interval (less than 7 days) in progressing to completed infarction.
Unilateral oculosympathetic palsy compels exclusion of an internal carotid artery dissection even in the absence of other signs or symptoms. The mechanism is related to compression of the third order postganglionic sympathetic fibers ascending along the internal carotid artery or ischemia of the vasa nervosum. The typical manifestations are miosis (constricted pupil) with dilatation lag, ptosis (drooping upper eyelid from loss of sympathetic innervation to the Müller, or superior tarsal muscle), and upside down ptosis (slight elevation of the lower lid). Anhidrosis is specifically not observed due to innervation of the facial sweat glands from the sympathetic plexus of the external carotid artery that is typically excluded from dissections.
Vertebral artery dissections are associated with posterior neck/occipital pain and posterior cerebral or brainstem/cerebellar ischemia. Although occipital neck pain may be easily dismissed for musculoskeletal symptoms, as many as 80% to 90% of patients will eventually develop ischemic sequelae from either flow limiting dissections, or more likely, thromboembolic events.
Brainstem/cerebellar ischemia from vertebral artery dissections is most frequently observed in the distribution of the affected posterior inferior cerebellar artery and commonly presents with lateral medullary (Wallenberg) syndrome. Dysfunction of the vestibular system, nucleus ambiguous, inferior cerebellar peduncle, lateral spinothalamic tract, or spinal trigeminal nucleus can result in nystagmus, diplopia, vertigo, nausea and vomiting, unilateral hearing loss, dysphagia, hoarseness, diminished gag reflex, ataxia, dysmetria, dysarthria and sensory deficits of the contralateral body and ipsilateral face. Occipital lobe ischemia in the posterior cerebral artery distributions may lead to bilateral visual deficits.
Less common manifestations of vertebral artery dissections include cervical radiculopathy from direct compression of the spinal nerve roots, spinal cord ischemia from compromise of the anterior and posterior spinal arteries or radiculomedullary artery (artery of cervical enlargement), and spinal epidural hematoma from hemorrhagic complications. Approximately 10% of vertebral artery dissections extend intracranially with the potential to rupture or form dissecting aneurysms, thereby presenting with subarachnoid hemorrhage.
Clinical presentation
Patients with cervical dissections present after minor or major inciting events resulting in neck hyperextension or rotation, often with sudden transitional or decelerating movements. Although dissections can occur following major head and neck trauma associated with motor vehicle accidents, many patients have a history of more subtle trauma such as practicing yoga, painting a ceiling, coughing, vomiting, sneezing, recent catheter angiography, anesthesia or resuscitation. In particular, chiropractic manipulation is notorious for causing carotid, and more frequently, vertebral artery dissections with 1 in 20,000 manipulations resulting in stroke.
Internal carotid artery dissections may present with the classic triad of ipsilateral headache or neck pain, partial Horner’s syndrome, and ischemic symptoms. However, lower cranial nerve palsies (particularly the hypoglossal nerve), impairment of taste (facial and glossopharyngeal nerve palsies), pulsatile tinnitus (or auscultation of a bruit), and cerebral and retinal transient ischemic attacks or infarcts can also be present at the time of initial presentation.
Nearly two thirds of patients with carotid dissections present with neck pain or headache usually described as a gradual onset of constant dull or aching pain, but acute presentations with severe throbbing, sharp pain, or even thunderclap headache may be seen. Anterolateral cervical pain associated with frontal-temporal headache or orbital/facial pain is usually the initial manifestation before ischemic symptoms. Similarly, transient ischemic attacks or transient monocular blindness can precede frank cerebral and retinal infarcts, with a relatively short time interval (less than 7 days) in progressing to completed infarction.
Unilateral oculosympathetic palsy compels exclusion of an internal carotid artery dissection even in the absence of other signs or symptoms. The mechanism is related to compression of the third order postganglionic sympathetic fibers ascending along the internal carotid artery or ischemia of the vasa nervosum. The typical manifestations are miosis (constricted pupil) with dilatation lag, ptosis (drooping upper eyelid from loss of sympathetic innervation to the Müller, or superior tarsal muscle), and upside down ptosis (slight elevation of the lower lid). Anhidrosis is specifically not observed due to innervation of the facial sweat glands from the sympathetic plexus of the external carotid artery that is typically excluded from dissections.
Vertebral artery dissections are associated with posterior neck/occipital pain and posterior cerebral or brainstem/cerebellar ischemia. Although occipital neck pain may be easily dismissed for musculoskeletal symptoms, as many as 80% to 90% of patients will eventually develop ischemic sequelae from either flow limiting dissections, or more likely, thromboembolic events.
Brainstem/cerebellar ischemia from vertebral artery dissections is most frequently observed in the distribution of the affected posterior inferior cerebellar artery and commonly presents with lateral medullary (Wallenberg) syndrome. Dysfunction of the vestibular system, nucleus ambiguous, inferior cerebellar peduncle, lateral spinothalamic tract, or spinal trigeminal nucleus can result in nystagmus, diplopia, vertigo, nausea and vomiting, unilateral hearing loss, dysphagia, hoarseness, diminished gag reflex, ataxia, dysmetria, dysarthria and sensory deficits of the contralateral body and ipsilateral face. Occipital lobe ischemia in the posterior cerebral artery distributions may lead to bilateral visual deficits.
Less common manifestations of vertebral artery dissections include cervical radiculopathy from direct compression of the spinal nerve roots, spinal cord ischemia from compromise of the anterior and posterior spinal arteries or radiculomedullary artery (artery of cervical enlargement), and spinal epidural hematoma from hemorrhagic complications. Approximately 10% of vertebral artery dissections extend intracranially with the potential to rupture or form dissecting aneurysms, thereby presenting with subarachnoid hemorrhage.
Diagnostic imaging
Ultrasound
Doppler ultrasound may be used for the initial screening and diagnosis of mid-cervical dissections, but its ability to assess the proximal carotid and vertebral arteries and distal cervical/intracranial vasculature is limited due to interference from the thoracic inlet, mandible, and skull base. Additionally, evaluation of the vertebral arteries is challenging as they course through the foramen tranversarium with specific findings detectable in only 20% of patients.
Although flow pattern abnormalities may be identified because of dissection related stenosis in more than 90% of patients, these are nonspecific findings demonstrating either decreased velocity with high resistance (stenosis), a biphasic pattern (occlusion), or compensatory elevated velocities. More specific findings include segmental dilatation, double lumen with echogenic flap, eccentric echogenic hematoma surrounding a narrowed arterial lumen, and low or absent flow velocity in the dissected false lumen. However, these findings are observed in less than one third of cases and most, if not all, patients will require further noninvasive cross-sectional imaging or conventional angiography.
Digital Subtraction Angiography
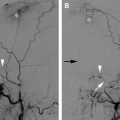
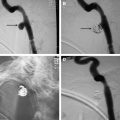
Digital subtraction angiography (DSA) has been considered the gold standard to diagnose or exclude cervical dissections. Along with three-dimensional (3D) rotational DSA techniques, its superior resolution allows the detection of subtle pathognomonic features such as intimal flaps or the double lumen sign, but these are seen in less than 10% of dissections. The most common angiographic finding is a string sign, smooth tapered (or slightly irregular) luminal narrowing in approximately 65% of patients that may progress to an abrupt tapered, flame-like internal carotid artery occlusion ( Fig. 1 A, F ). A characteristic location is 2 to 3 cm distal to the carotid bulb with variable extension into the mid or distal cervical segment of the internal carotid artery, though migration is limited by the osseous foramen of the petrous carotid canal. In the vertebral artery, the V1 and V3 segment at the points of entry (C6-C7) and exit (C1-C2 loops) from the foramen transversarium also appear to be common locations for cervical dissections. Location of the pathology and involvement of long segments in cervical dissections assists in differentiation from atherosclerotic disease.
Subadventitial dissections result in dissecting aneurysms or pseudoaneurysms in 25% to 35% of patients. The dissecting aneurysms are often fusiform or oval in shape and extend parallel to the arterial lumen. Termed a pearl and string sign, dissecting aneurysms are often seen at the distal margin of a stenotic segment on DSA. Rupture of a mid-cervical dissecting aneurysm may cause the formation of carotid-cutaneous or carotid-pharyngeal fistula with contrast extravasation and arteriovenous shunting best identified on DSA imaging.
Other angiographic findings may include distal thromboembolic branch occlusions of the intracranial arteries that may be difficult to appreciate on cross-sectional imaging. In addition, DSA accurately depicts flow directionality and intracranial transit time, and remains the best modality to assess the collateral intracranial circulation. DSA is superior to cross-sectional imaging studies in evaluating flow-limiting dissections, dissections complicated by arteriovenous fistulas, and flow remodeling after stent placement.
Although DSA is the optimal technique to assess the arterial lumen, its major limitation is its inability to directly image the vessel wall. Therefore, it may be inferior to cross-sectional techniques in the evaluation of nonstenotic dissections with benign intramural hematomas or thrombosed pseudoaneurysms. Another disadvantage of DSA is its potential for ischemic complications. However, the incidence of major neurologic complications from DSA is very low if it is performed by experienced and well-trained physicians. Since multiple vessel dissections occur in up to 25% of patients, careful diagnostic analysis should include bilateral carotid and vertebral artery injections with proximal catheter positioning (proximal common carotid or subclavian arteries) to prevent further injury of a dissected vessel segment and to visualize the entire cervical course and intracranial vasculature.
Computed Tomography Angiography
Noncontrast CT may be useful in evaluating thromboembolic complications of dissections, subacute infarcts, or subarachnoid hemorrhage from intracranial dissections or dissecting aneurysms. CTA is rapidly emerging as a highly sensitive and specific modality for large and medium vessel pathologies of the head and neck. Multi-detector technology enables capture of peak contrast enhancement yielding exceptional detail to evaluate the vessel lumen in addition to the vessel wall. Axial thin-section (0.625 mm–1.25 mm) scanning with enhanced postprocessing capability allows multiplanar reformatted images, 3D reconstructions, and curved maximum intensity projections in virtually any orientation of the vessel ( Fig. 1 B). In a recent study comparing CTA and MRA, Virtinsky and colleagues show CTA to be the preferred cross-sectional modality to delineate the imaging features of cervical dissections, especially for vertebral artery dissections. CTA imaging provides accurate measurements of vessel lumen diameter and dissection length and can provide useful information in treatment planning if endovascular stent reconstruction is planned.
In the carotid or vertebral arteries, CTA findings of an irregular, narrowed contrast enhancing lumen are indicative of dissection. Vessel wall thickening is often identified on CTA from a subintimal or intramural hematoma and it frequently corresponds with a methemoglobin crescent sign on MRA. A discrete intimal flap or patent double lumens are rarely seen findings ( Fig. 1 D, E). Dissecting aneurysms may be readily identified as focal outpouchings of the enhancing arterial lumen, with or without associated thrombus, and often in an orientation parallel to the vessel.
A relative limitation of CTA imaging is difficulty in its interpretation when extensive calcifications are present along the arterial wall. Furthermore, streak and beam hardening artifact from a patient’s dental hardware may limit evaluation of the midcervical internal carotid artery, a commonly involved site for dissections. Although recent advances in dynamic CTA with 256 and 320 multislice CT scanners may capture both arterial and venous phases, at this time, flow directionality and transit times are best analyzed on DSA.
MR Imaging and MR Angiography
MR imaging and MRA remain the initial screening modality of choice to evaluate patients with suspected cervical dissections. MR imaging with fat saturated axial T1 and T2 weighted sequences provides a sensitive technique to identify subtle dissections where no significant luminal narrowing or mural thickening may be appreciated on DSA or CTA. In fact, the periluminal (subintimal or intramural) hematoma may be uniquely identified on MR imaging in the subacute stage (3–14 days).
The classic finding is circumferential or crescentic hyperintense signal peripheral to the flow void of an irregularly narrowed internal carotid or vertebral artery. Depending on the stage and composition of the hemorrhagic products, the signal on T1 and T2 weighted sequences may vary. In the acute stage (1–3 days), deoxyhemoglobin (T1 isointense and T2 hypointense) may not be apparent and can be difficult to detect. Conversely, methemoglobin in the early subacute stage (3–7 days) causes T1 shortening (T1 hyperintensity) that is easily recognizable against the adjacent flow void. Extracellular methemoglobin in the late subacute stage (7–14+ days) demonstrates T1 and T2 hyperintensity also facilitating detection ( Fig 2 A , B). Fat suppression techniques are invaluable in differentiating periluminal hematoma from the periarterial fat. Recent studies at our institution suggest that presence of restricted diffusion on diffusion weighted imaging (DWI) of the neck may also provide increased sensitivity for detection of intramural hematomas and detection of cervical dissections ( Fig. 2 C, D) (H. Parmar, unpublished data, 2008). Additionally, since both head and neck imaging are routinely performed, screening for ischemic intracranial complications can be simultaneously performed using DWI.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree