Chapter 3 Challenge
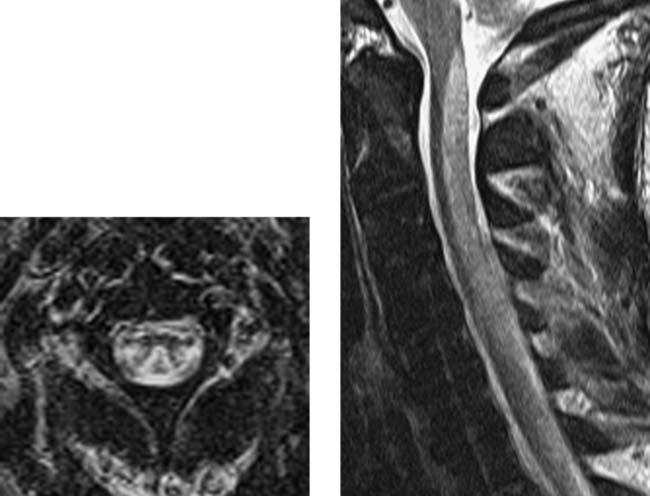
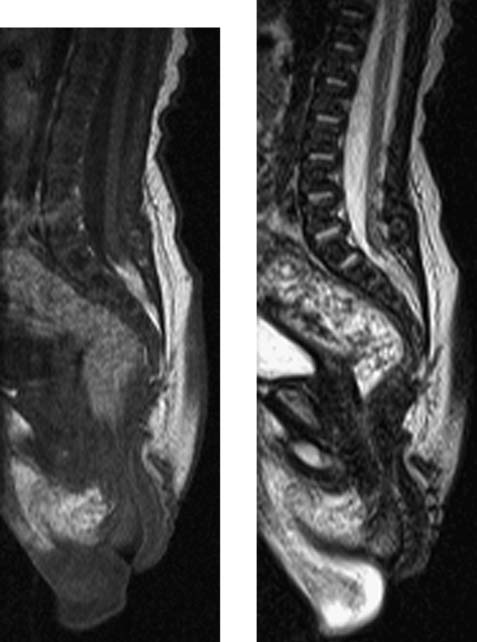
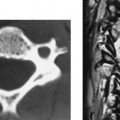
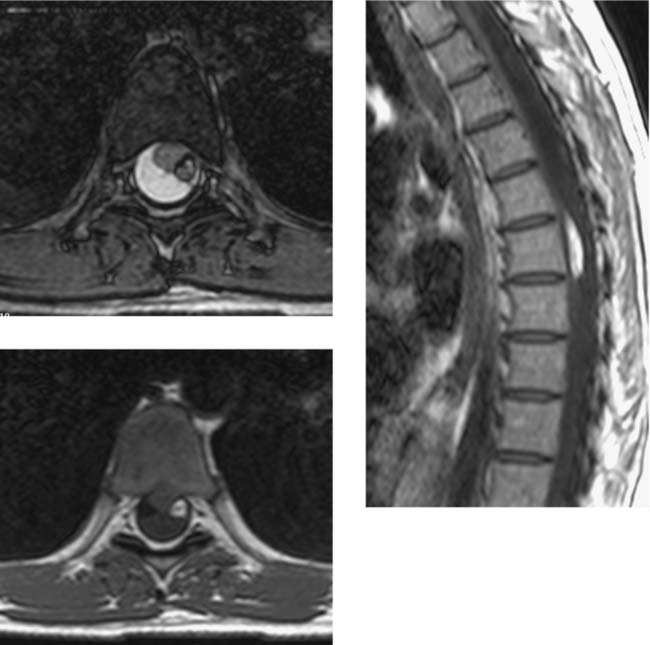
Intradural Myolipoma, Thoracic
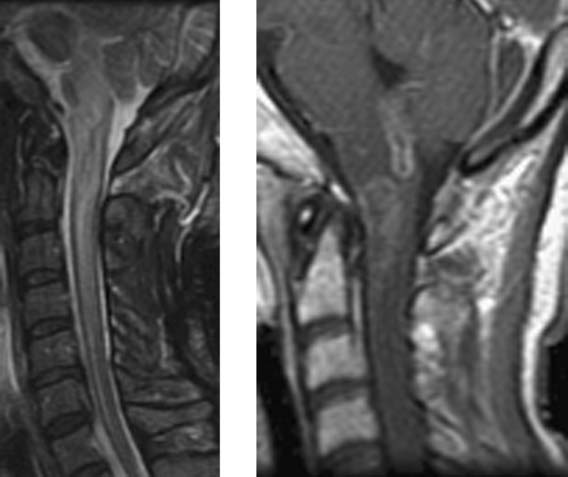
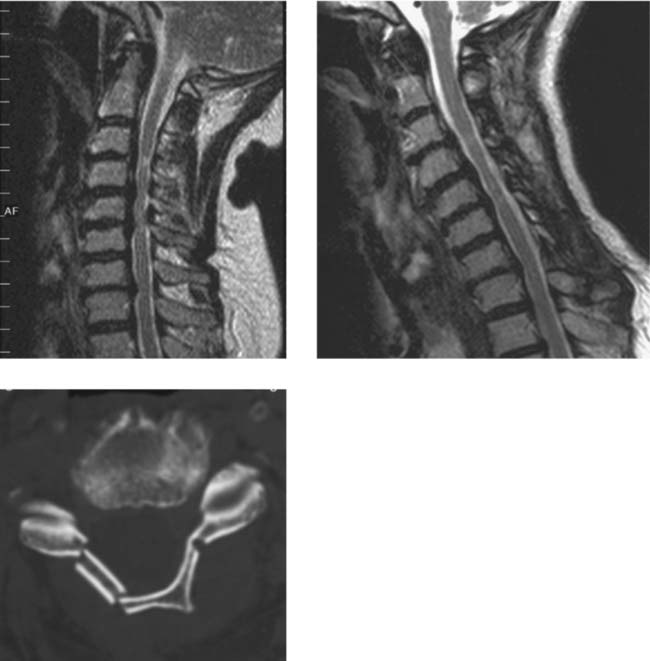
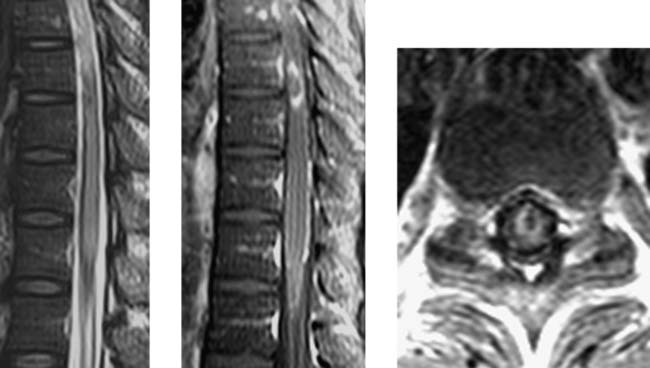
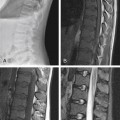
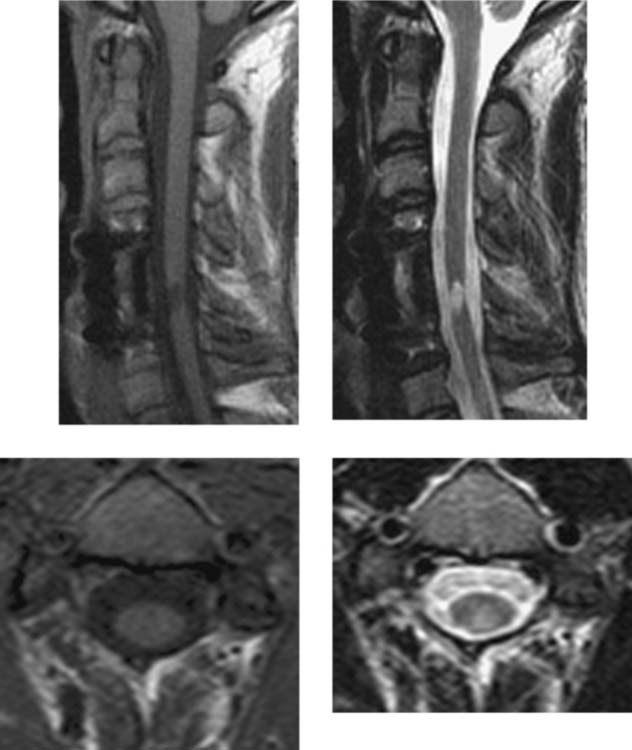
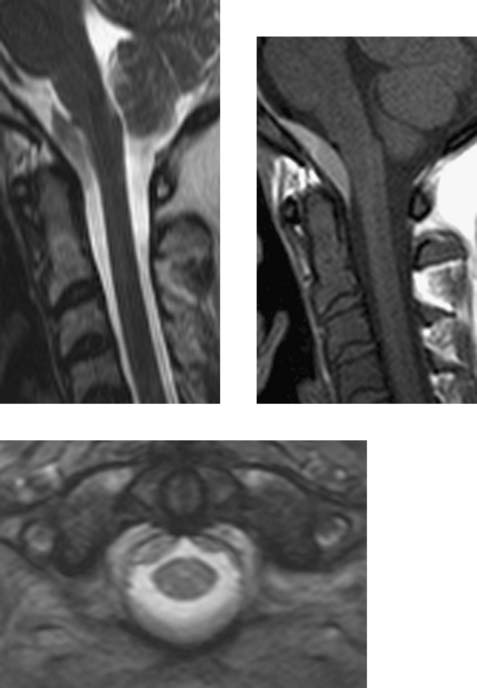
Wallerian Degeneration, Cervical
Becerra JL, Puckett WR, Hiester ED, et al. MR-pathologic comparisons of Wallerian degeneration in spinal cord injury. AJNR Am J Neuroradiol. 1995;16:125-133.
Buss A, Pech K, Merkler D, et al. Sequential loss of myelin proteins during Wallerian degeneration in the human spinal cord. AJNR Am J Neuroradiol. 2005;128:356-364.
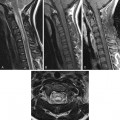
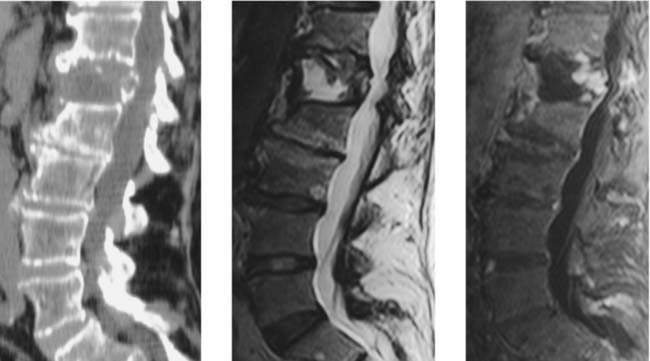
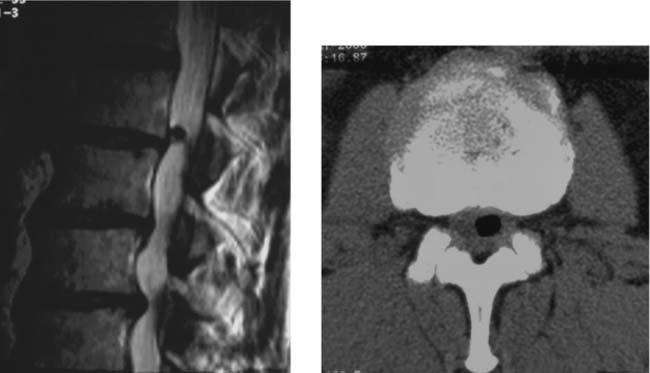
Vertebral Body Avascular Necrosis
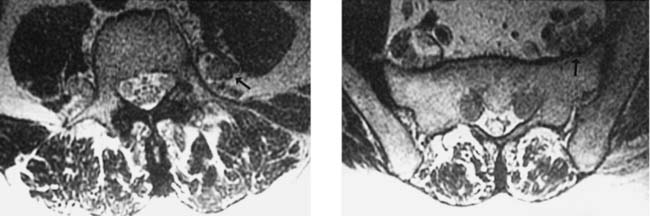
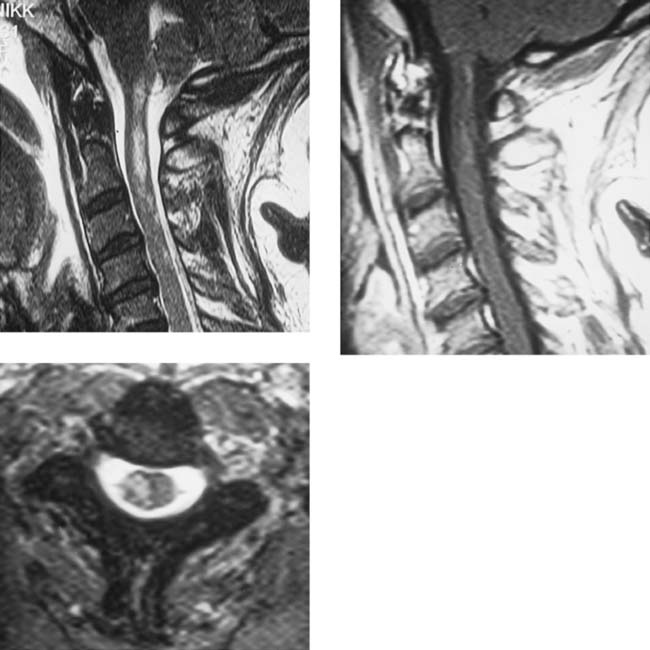
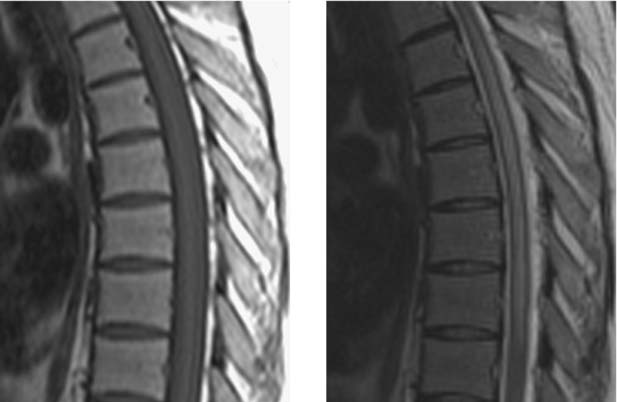
Unilateral Cord Infarction, Cervical
Bergqvist CA, Goldberg HI, Thorarensen O, Bird SJ. Posterior cervical spinal cord infarction following vertebral artery dissection. Neurology. 1997;48:1112-1115.
Laufs H, Weidauer S, Heller C, et al. Hemi-spinal cord infarction due to vertebral artery dissection in congenital afibrinogenemia. Neurology. 2004;63:1522-1523.
Acute Spinal Subdural Hematoma
Morris SF, Poynton AR, O’Donnell T, McCormack D. Lumbosacral subdural hematoma following minor trauma: a case report. J Bone Joint Surg Am. 2004;86:1768-1771.
Post MJD, Becerra JL, Madsen PW, et al. Acute spinal subdural hematoma: MR and CT findings with pathologic correlates. AJNR Am J Neuroradiol. 1994;15:1895-1905.
Intraspinal Gas Collection
Radiation Myelopathy
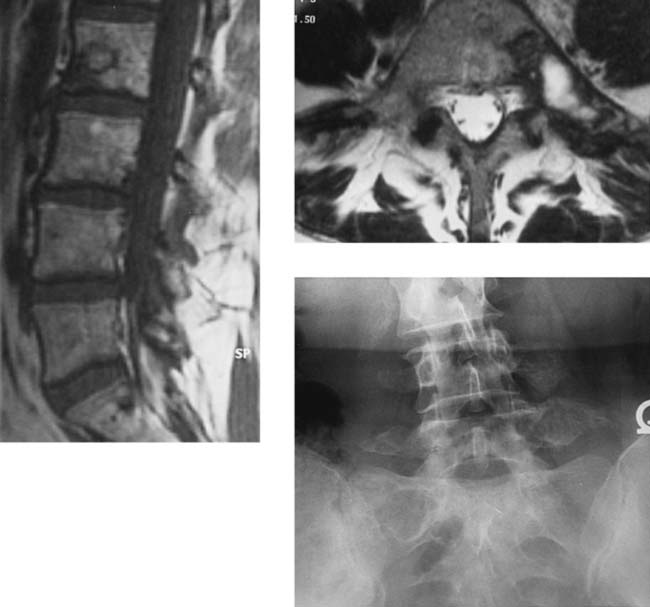
Polyostotic Fibrous Dysplasia
Chow LT, Griffith J, Chow WH, et al. Monostotic fibrous dysplasia of the spine: report of a case involving the lumbar transverse process and review of the literature. Arch Orthop Trauma Surg. 2000;120:460-464.
Leet AI, Magur E, Lee JS, et al. Fibrous dysplasia in the spine: prevalence of lesions and association with scoliosis. J Bone Joint Surg Am. 2004;86-A:531-537.
Subacute Combined Degeneration (SCD)
Ravina B, Loevner LA, Bank W. MR findings in subacute combined degeneration of the spinal cord: a case of reversible cervical myelopathy. AJR Am J Roentgenol. 2000;174:863-865.
Yamada K, Shrier DA, Tanaka H, Numaguchi Y. A case of subacute combined degeneration: MRI findings. Neuroradiology. 1998;40:398-400.
Listeria Myelitis/Rhombencephalitis
Alper G, Knepper L, Kanal E. MR findings in listerial rhombencephalitis. AJNR Am J Neuroradiol. 1996;17:593-596.
Mendonca RA. Spinal infection and inflammatory disorders. In: Atlas SW, editor. Magnetic Resonance Imaging of the Brain and Spine. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2002:1919-1920.
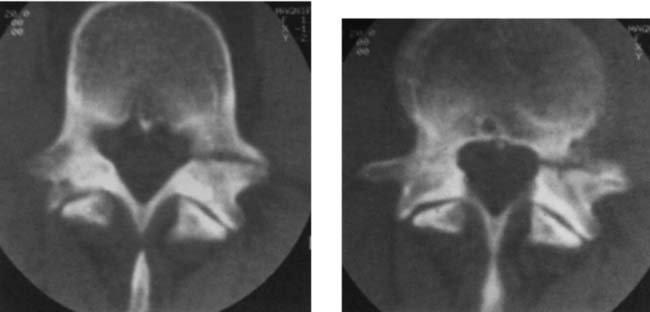
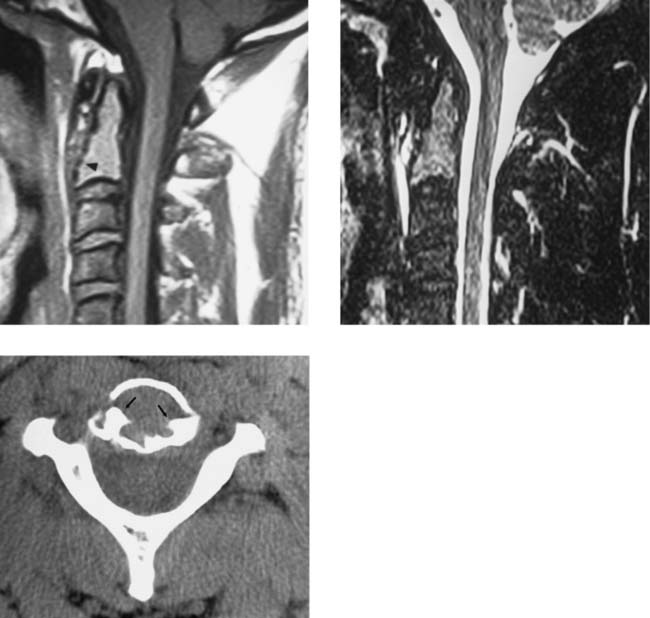
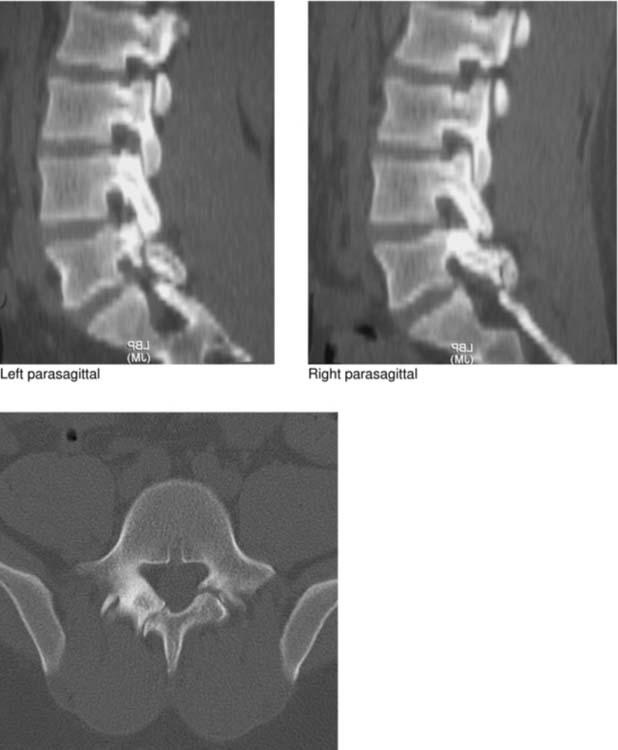
Retrosomatic Cleft
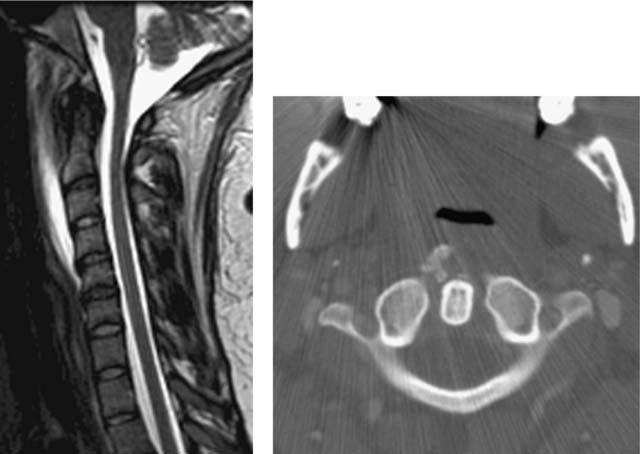
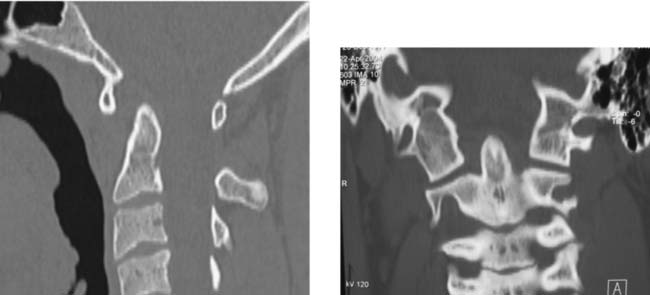
Acute Calcific Prevertebral Tendinitis (Calcific Tendinitis of the Longus Colli Muscle)
Eastwood JD, Hudgins PA, Malone D. Retropharyngeal effusion in acute calcific prevertebral tendinitis: diagnosis with CT and MR imaging. Am J Neuroradiol. 1998;19:1789-1792.
Rosbe KW, Meredith SD. Imaging quiz case 2: calcific tendinitis of the longus colli muscle. Arch Otolaryngol Head Neck Surg. 2000;126:1031-1035.
Charcot-Marie-Tooth Disease (Hereditary Motor and Sensory Neuropathy Type I)
Expansive Open-Door Laminoplasty
Plasmacytoma
Vestigial Tail
Retroisthmic Cleft
Atlantoaxial Dislocation with Occipitalized Atlas
Intramedullary Abscess
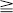 thoracic
thoracic 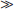 sacral
sacral 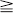 cervical (see Leet et al). Monostotic FD of the spine is exceedingly rare (< 30 cases), usually involves the vertebral body and adjacent pedicle, and shows no predilection for a particular spinal region. Most spinal FD lesions are asymptomatic and require no treatment. Many patients do have spinal pain, and a few pathologic compression fractures, with or without trauma, have been reported. Scoliosis is common in patients with polyostotic FD.
cervical (see Leet et al). Monostotic FD of the spine is exceedingly rare (< 30 cases), usually involves the vertebral body and adjacent pedicle, and shows no predilection for a particular spinal region. Most spinal FD lesions are asymptomatic and require no treatment. Many patients do have spinal pain, and a few pathologic compression fractures, with or without trauma, have been reported. Scoliosis is common in patients with polyostotic FD.