Although ultrasound is the initial imaging modality of choice in patients with right upper quadrant pain or suspected biliary obstruction, a number of challenges in clinical practice limit its utility as a stand-alone imaging modality. This article presents a focused review of gallbladder and biliary ultrasound, highlighting current knowledge gaps, emerging applications, and directions for further study. The authors cover selected topics including acute cholecystitis, cystic artery velocity, gallbladder polyps, contrast-enhanced ultrasound, and incidental biliary duct dilatation.
Key points
- •
While ultrasound (US) is fundamental for assessment of acute cholecystitis, its measured performance has declined and would benefit from standardization in interpretation and reporting.
- •
Cystic artery velocity is a promising technique in diagnosis of acute cholecystitis but lacks supporting data and requires further study to define its clinical role and utility.
- •
Recent Society of Radiologists in Ultrasound consensus management guidelines for commonly identified, incidental gallbladder polyps aim to reduce unnecessary follow-up and surgery, but still require prospective and systematic evaluation.
- •
Lack of enhancement on contrast-enhanced US has high negative predictive value for excluding blood flow and is more useful than color Doppler evaluation for differentiating tumefactive sludge from gallbladder neoplasm.
- •
Incidental biliary duct dilatation is common in clinical practice. A recently presented algorithmic approach incorporating liver chemistries can help determine which patients need further evaluation.
Introduction
Ultrasound (US) is generally considered the initial imaging modality of choice in patients with right upper quadrant pain or suspected biliary obstruction. It uses no ionizing radiation, is cost-effective, and can be performed portably in patients who are unstable. Real-time imaging allows for assessment of the mobility of intraluminal structures and presence of a sonographic Murphy sign (SMS). Furthermore, it has the highest sensitivity of any modality for the diagnosis of cholelithiasis. Important limitations of US include its operator dependence and potential for image degradation by larger patient body habitus or intervening bowel gas.
Despite the ubiquitous role of US in the evaluation of the gallbladder and biliary system, a number of challenges in clinical practice limit its utility as a stand-alone imaging modality. Accordingly, US is considered a highly effective screening test, but the presence of positive or indeterminate findings on US frequently prompt further evaluation with computed tomography (CT), magnetic resonance imaging with cholangiopancreatography (MRI/MRCP), or hepatobiliary scintigraphy. In the current article, the authors provide a focused review of the literature, highlighting current knowledge gaps and future directions for US of the gallbladder and bile ducts. Selected topics include routine evaluation of acute cholecystitis (AC), cystic artery velocity (CAv) for the diagnosis of AC, incidental gallbladder polyps, contrast-enhanced US (CEUS), and incidental biliary duct dilatation.
Ultrasound of Acute Cholecystitis
The clinical diagnosis of AC relies on physical examination signs localizing inflammation to the right upper quadrant, systemic signs of inflammation and confirmatory imaging findings. The imaging findings of AC include confirmation of gallstones in appropriate patients and various secondary findings of gallbladder inflammation or obstruction. Multiple imaging modalities are used to confirm AC including US, hepatobiliary scintigraphy, CT and MRI/MRCP. US is widely considered the most appropriate initial test because of its high accuracy, low cost, and broad availability. Although early studies found US to have positive and negative predictive values for AC exceeding 90%, its reported performance has declined substantially in more recent large meta-analyses, with pooled sensitivity and specificity of 81% and 83%, respectively, in 2012, and 69% and 79%, respectively, in 2023. Meanwhile, recent data suggest that CT and MRI have comparable or superior performance to US in the diagnosis of AC. The explanations for and implications of these evolving performance metrics are not yet understood.
Detection of gallstones with US is a well-established and usually straightforward component of the US diagnosis of AC. The role of secondary findings–how they are defined, their relative significance, and how many are necessary or sufficient to make a confident diagnosis–remains surprisingly ambiguous, however. The best-established secondary US findings are the “SMS” and thickening of the gallbladder wall. Additional secondary findings include gallbladder distension and identification of an impacted stone within the gallbladder neck ( Fig. 1 ).

The SMS, first reported in the late 1970s as an elaboration on the widely accepted SMS, was arguably the first reported secondary US finding of AC. Early studies reported a positive predictive value of 92% in association with stones and a negative predictive value, absent stones, of 95%. It was later recognized that the SMS may be absent in gangrenous cholecystitis due to ischemic denervation of the gallbladder. The first definitions of the SMS were “the point of maximal abdominal tenderness over the sonographically localized gallbladder,” noting “…if there was no tenderness over the gallbladder, or if tenderness was the same over the gallbladder as in adjacent regions, [the SMS] was negative.” , Key features of the SMS were therefore “tenderness,” an objective sign assessed by the examiner (as opposed to “pain,” a symptom reported by the patient) and a deliberate comparison of tenderness at multiple sites on the abdomen. However, descriptions of the SMS have varied widely in terms of how tenderness or pain is defined and assessed. , This variation and the evolution of radiology practice since the late 1970s toward near-universal scanning by sonographer technologists with remote image interpretation by radiologists may account for decreased performance of this feature. Assessment of the SMS in the setting of opioid analgesia also presents an increasingly common ambiguity. When a sonographer is unfamiliar with the patient’s baseline prior to medication, or if the sonographer is not trained or experienced in assessment of objective signs of abdominal tenderness, the SMS may be less reliable. A prospective study of emergency room patients randomized to receive placebo or meperidine reported that the performance of the SMS was unaffected by meperidine when assessed by emergency providers trained in bedside US, but that sensitivity of the SMS was as low as 17% when obtained by sonographers after medication was given, with many sonographers noting that the SMS could not be assessed. The same authors noted an increasing trend among emergency providers toward early prescription of analgesics to patients with suspected AC.
After the SMS, gallbladder wall thickening is the other major, longest established secondary US finding of AC. Defining abnormal thickness as greater than 2 mm, a widely cited early study showed a high positive predictive value of 95% in association with gallstones. A thickness greater than 3 mm is now widely considered to be the threshold for abnormal, , although proposed thresholds have varied from 2 to 5 mm. As with the SMS, there is a lack of consistency in the literature regarding where and how the gallbladder wall should be measured, or how to deal with partial or focal wall thickening. Gallbladder wall edema has also been described variably in the literature, but its distinction as a feature from wall thickening is not clear, with some authors describing a distinct, “hypoechoic layer” in the gallbladder wall and others describing a “striated appearance” of the wall in association with gangrenous cholecystitis. , There are also a wide variety of conditions other than cholecystitis that can cause gallbladder wall thickening, limiting the specificity of this finding in patients with chronic liver disease, acute hepatitis, heart failure, and many other conditions. Notably, early studies of this finding excluded patients with ascites and only included positive patients referred specifically for evaluation of suspected biliary disease, likely a very different population than those undergoing evaluation of abdominal pain in the emergency department today.
Several other secondary findings of AC have been described but are generally less supported by data and are variably applied in practice. Distension of the gallbladder lumen, for example, often defined as a luminal width greater than 4 cm, is a common secondary finding of AC. However, the gallbladder may also be distended in the fasting state, a typical scenario in patients with abdominal pain. Gallbladder size at baseline can also vary substantially from person to person. Due to these factors, defining gallbladder distension by a specific diameter or size threshold has limited positive predictive value, as low as 65%. The tense gallbladder fundus sign has also been described as a marker of gallbladder distention and/or obstruction on CT ; however, no equivalent findings have been described on US. Notably, the absence of gallbladder distension using a luminal diameter threshold of less than 2.2 cm is likely to exclude AC.
Direct visualization of an obstructing stone in the gallbladder neck or cystic duct and demonstration of its non-mobility has been proposed as another secondary finding, but has not been studied extensively. An impacted stone in the neck is identified by absence of anechoic bile between the stone and the walls of the gallbladder neck, and does not displace from the neck when moving the patient from supine to left lateral decubitus to nearly prone position. In patients with a gallbladder-oriented steeply in the cranio-caudal axis, moving the patient to an upright or even standing position may be necessary to displace the stone.
Despite the emergence of these and other secondary findings omitted from this discussion, there is no consensus as to their relative significance in making the diagnosis. The only available meta-analyses of US performance for AC describe extensive heterogeneity among studies regarding how many findings were considered diagnostic, representing a significant limitation. As illustrated earlier, there is and has always been considerable ambiguity in assignment of even the most fundamental secondary features of AC. In combination with evolving practice in radiology and emergency departments, this ambiguity has likely contributed to the declining performance of US in diagnosis of AC. Focused training for sonographers in assessment of the SMS, especially in the setting of prior analgesia, as well as direct involvement by the radiologist whenever possible in ambiguous cases, will likely improve the utility of this important secondary finding. A comprehensive guidance system, akin to the Liver Reporting & Data System, to establish universal standards for interpretation and reporting, would also likely improve the performance of US in practice and facilitate clinical research and data collection. Finally, incorporation of novel techniques like CAv, as detailed in the subsequent section, provides further potential for improvement.
Cystic Artery Velocity
Pericholecystic inflammatory changes that occur in AC are commonly visualized on hepatobiliary scintigraphy as the “hot rim” sign, and on contrast-enhanced CT or CEUS as hyperemia of the hepatic parenchyma adjacent to the gallbladder fossa. , Correlations have been similarly described between increased color or power Doppler signal within the gallbladder wall and AC in the older US literature. With routine use of spectral Doppler, quantitative parameters of pericholecystic hyperemia have essentially replaced qualitative assessment, which is notoriously dependent on the color Doppler settings which are utilized (eg, gain, frequency, range).
Both peak hepatic artery velocity (HAv) and CAv have been associated with AC in various reports in the literature and with variable performance. In a series of patients, 21 with AC and 14 with chronic cholecystitis (CC), peak HAv 100 cm/s or greater had an accuracy of 69% for the diagnosis of AC. In a larger recent study which included 119 patients with AC and 117 with CC, a simple model incorporating peak HAv 96 cm/s or greater, gallbladder distention, and wall abnormalities had an area under the operating curve of 0.75, and sensitivity and specificity of only 60% and 83%, respectively, for differentiating AC from CC. Increased peak HAv is not specific for AC, nor is it necessarily specific to primary hepatobiliary disease. Accordingly, an evaluation of a series of patients with peak HAv 200 cm/s or greater found that elevations could be attributed to a variety of causes including generalized infection and sepsis.
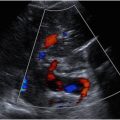
Peak CAv, on the other hand, is likely more specific than peak HAv in the evaluation for pericholecystic inflammation ( Fig. 2 ). An older study found that a peak CAv of greater than 40 cm/s was indicative of AC in patients with cirrhosis and a thickened gallbladder wall, though only 6 patients with AC were included. In a recent study performed by Perez and colleagues, peak CAv was evaluated in 43 patients who presented to the emergency department with right upper quadrant pain, 25 with AC and 18 with CC, as well as in 30 control patients. The authors found that a peak CAv 40 cm/s or greater was highly associated with AC, with positive predictive value of 95% and an overall accuracy 81% when used alone. However, this study was performed retrospectively, and the majority of patients screened for inclusion were excluded either because CAv was not measured, or they did not undergo definitive treatment within 6 days after imaging.

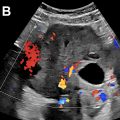
In our practice, we have encountered a number of patients with elevated peak CAv but who do not have AC, which may occur from variety of factors including hyperdynamic state, shock, hepatitis, and pancreatitis ( Fig. 3 A, B ). Spuriously normal peak CAv, on the other hand, may occur in early AC or low cardiac output states ( Fig. 3 C, D). Further study is needed to determine the true performance CAv in practice and define its utility in combination with other more established features of AC. CAv will likely serve as a tiebreaking feature if grayscale findings are equivocal and/or SMS cannot be elicited.