Chest, Thyroid, Parathyroid, and Neonatal Brain Ultrasound
William E. Brant
Chest
US is an excellent supplement to conventional radiography and CT for the problem-solving evaluation of the chest and to guide interventional procedures in the thorax (1,2,3,4). US can image into and through pleural effusions and lung consolidation to evaluate the thorax opacified on plain radiographs. Its portability allows the evaluation of critically ill patients who are impractical to move for a CT. US examination of the chest must always be correlated with the available chest radiography.
Pleural Space
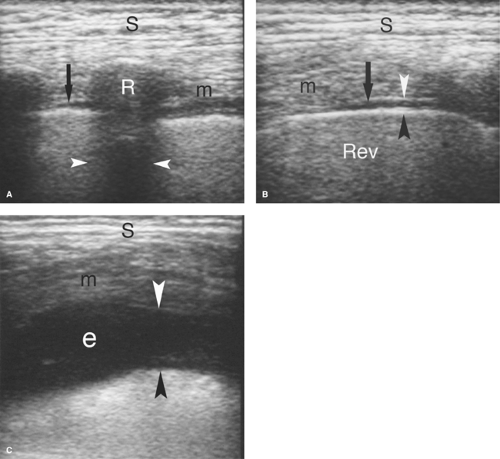
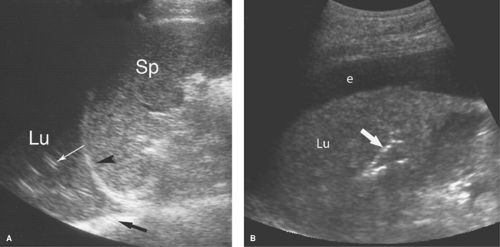
Normal US Anatomy. Air in the lungs completely reflects the US beam and prohibits examination deeper into the chest. However, when pleural fluid displaces air-filled lungs away from the chest wall, disease in the pleural space can be optimally evaluated with US. The pleural space is examined by a direct intercostal approach with the US transducer applied directly to the chest, or by an abdominal approach imaging through the diaphragm from the abdomen. The ribs are used as sonographic landmarks for direct chest imaging (Fig. 38.1). A linear array transducer applied to the chest wall shows the ribs as curving echoes that cast acoustic shadows. The visceral pleura–air-filled-lung interface is seen within 1 cm of the rib echo as a bright echogenic surface that moves with respiration (the “gliding sign”). The moving lung surface is well visualized when the transducer is turned to parallel the intercostal space. The tiny normal amount of fluid in the pleural space is seen just superficial to the gliding pleura. From the abdomen, the diaphragm is seen as a bright curving interface due to complete sound reflection from the air-filled lung above it (Fig. 38.2). Organs beneath the diaphragm (liver and spleen) are artifactually reproduced above the diaphragm due to multipath sound reflection (the “mirror-image” artifact).
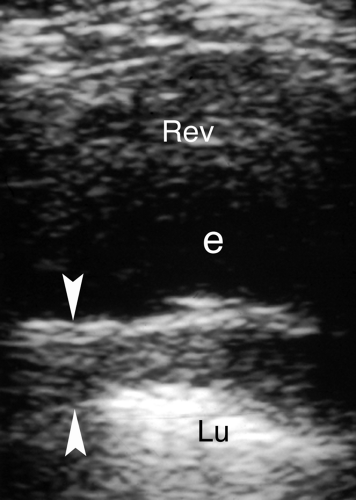
Pleural fluid displaces the lung away from the chest wall, allowing visualization of the pleural space (Figs. 38.1C, 38.2B). Most pleural fluid is anechoic or hypoechoic with floating particulate matter (7). The fluid separates the visceral and parietal pleural surfaces. From an abdominal approach, hypoechoic fluid is seen above the diaphragm, the inside of the thorax is visualized, and the mirror-image artifact is not present. Septations not evident on CT are commonly visualized by US. Collapsed or consolidated lung moves with respiration within the fluid in the pleural space. Fluid that is echogenic, contains floating particles or layering debris, or is septated is an exudate (Fig. 38.3). Fluid that is anechoic may be a transudate, exudate, or even empyema. Loculations of pleural fluid and suspected empyemas can be localized and evaluated, with US visualization used to guide needle aspiration and drainage catheter placement.
Pleural thickening complicates inflammatory and malignant disease of the thorax. US demonstrates uniform, undulating, or plaque-like thickening of the pleura (Fig. 38.4). The visceral pleura is easily evaluated. The parietal pleura is partially obscured by reverberation artifact in the near field.
Pleural Masses. Pleural metastases or tumors such as mesotheliomas are seen as nodular pleural thickening or hypoechoic soft-tissue masses in the pleural space projecting from the pleural surface.
Pneumothorax can be diagnosed by US (5). Pneumothorax produces a highly echogenic reflective line very similar to that of air-filled lung but lacking the “gliding sign” associated with respiratory movement. Pneumothorax is also indicated by loss of visualization of a previously visualized lung lesion that occurs during an invasive procedure.
Lung Parenchyma
Normal US Anatomy. The normal air-filled lung with its covering visceral pleura completely blocks the transmission of US into the thorax. The gliding visceral surface of the lung is easily seen, but reverberation artifact is displayed deep to that surface. However, consolidation, atelectasis, or tumor that extends
to the visceral pleural surface produces a window for US examination. When scanning the thorax from the abdomen, the normal air-filled lung produces a mirror-image artifact.
to the visceral pleural surface produces a window for US examination. When scanning the thorax from the abdomen, the normal air-filled lung produces a mirror-image artifact.
Consolidation refers to the filling of the air spaces of the lung with fluid and inflammatory cells. This process
“solidifies” the lung and provides a medium for sound transmission (Fig. 38.5). The consolidated lung appears solid and hypoechoic with echogenicity similar to that of the liver. Sonographic air bronchograms and sonographic air alveolograms may be seen within the consolidated lung. Air-filled bronchi produce bright branching linear reflections. Air trapped in the alveoli surrounded by consolidated lung produces globular bright echoes with comet-tail artifacts. Sonographic fluid bronchograms appear as anechoic fluid-filled tubes extending from the hilum of the lung. Color flow US demonstrates pulmonary vessels extending through the consolidated lung.
“solidifies” the lung and provides a medium for sound transmission (Fig. 38.5). The consolidated lung appears solid and hypoechoic with echogenicity similar to that of the liver. Sonographic air bronchograms and sonographic air alveolograms may be seen within the consolidated lung. Air-filled bronchi produce bright branching linear reflections. Air trapped in the alveoli surrounded by consolidated lung produces globular bright echoes with comet-tail artifacts. Sonographic fluid bronchograms appear as anechoic fluid-filled tubes extending from the hilum of the lung. Color flow US demonstrates pulmonary vessels extending through the consolidated lung.
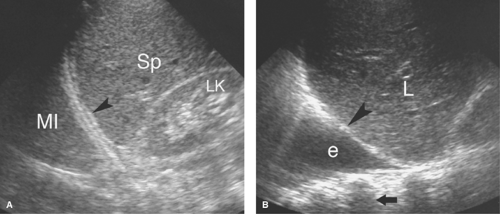
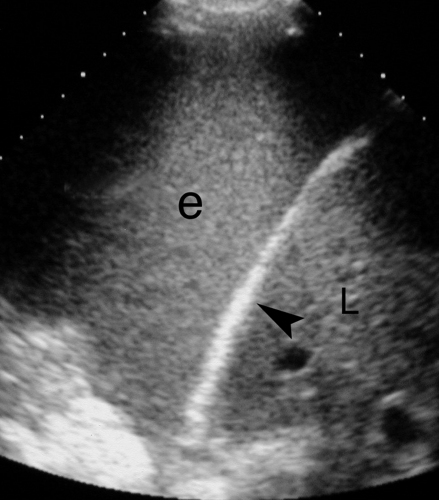
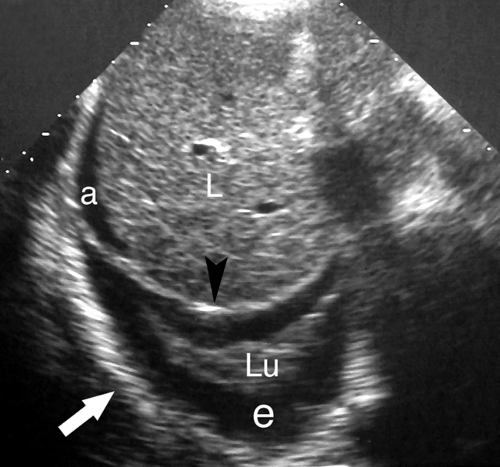
Atelectasis. Collapse of the air spaces with absorption of air also results in solidification of the lung. With atelectasis, the lung volume is decreased and bronchi and pulmonary blood vessels are crowded together. Collapsed lung always accompanies large pleural effusions (Fig. 38.6). The atelectatic lung is wedge shaped and is sharply defined by its covering pleura.
Lung masses completely surrounded by air-filled lung are not visualized by US, but those that extend to the visceral pleura or are accompanied by peripheral consolidation or atelectasis may be seen and evaluated (Fig. 38.7). US guidance may be effectively used to aspirate or biopsy lung masses in areas difficult to access with CT or fluoroscopy. Central tumor necrosis, hemorrhage within tumors, and lung abscesses are effectively evaluated.
Pulmonary sequestration is a congenital partition of lung tissue that does not communicate with the bronchial tree. Most occur at the lung base. Intralobar sequestrations are within the visceral pleura. Extralobar sequestrations are invested by their own separate pleura. US is used to confirm the diagnosis by the demonstration of a feeding artery arising from the aorta. Extralobar sequestrations drain via a systemic vein, whereas intralobar sequestrations connect to the pulmonary veins.
Mediastinum
Normal US Anatomy. The superior and anterior mediastinum is effectively evaluated with US using a parasternal or supramanubrial approach. The posterior mediastinum is less accessible because of spine and lung. Large lesions create sonographic windows to the mediastinum. Imaging downward into the superior mediastinum from just above the sternal manubrium demonstrates the innominate veins and the arteries arising from the aortic arch. Doppler US assists in the identification of vessels.
Vascular Lesions. Elongation and tortuosity of the brachiocephalic artery is a common cause of mediastinal widening in older adults. This diagnosis is easily confirmed by US, which can also exclude other masses of the superior mediastinum.
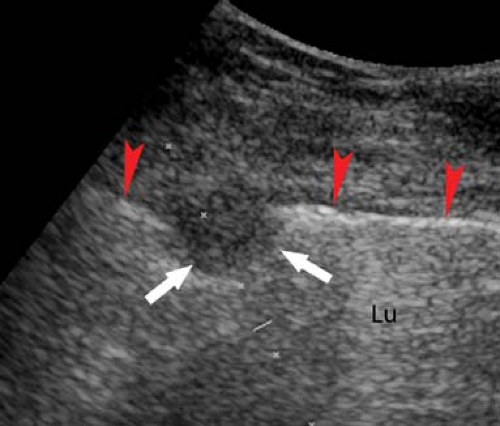
Mediastinal Masses. Thymic masses, substernal extension of thyroid enlargement, adenopathy, and other mediastinal masses are effectively demonstrated by US, which can confirm their cystic or solid nature and vascularity. Lesions that can be visualized by US can usually be biopsied using US guidance to avoid critical structures (Fig. 38.8). Continuation of thyroid tissue into the mediastinum is a straightforward diagnosis. Enlarged lymph nodes are usually homogeneous and hypoechoic. Confluent adenopathy due to lymphoma produces a solid, homogeneous, hypoechoic mass that encompasses and displaces blood vessels.
Thyroid
Imaging of the thyroid gland is a controversial topic (6,7,8). Thyroid nodules are exceedingly common, although thyroid cancer is uncommon and death from thyroid malignancy is rare. High-resolution US is extremely sensitive in detecting thyroid nodules; however, imaging signs to differentiate benign from malignant lesions overlap and are of limited sensitivity and specificity (9,10). Incidental discovery of thyroid nodules on CT or MR studies obtained for other reasons contributes to a current epidemic of thyroid nodules. This creates a recurring clinical problem of what to do with the many nodules
detected. In 2005, the Society of Radiologists in Ultrasound (SRU) published a consensus statement thought to represent a reasonable approach to nodular thyroid disease (11). In 2007, additional recommendations have been made on fine-needle aspiration (FNA) of thyroid nodules by the National Cancer Institute (12).
detected. In 2005, the Society of Radiologists in Ultrasound (SRU) published a consensus statement thought to represent a reasonable approach to nodular thyroid disease (11). In 2007, additional recommendations have been made on fine-needle aspiration (FNA) of thyroid nodules by the National Cancer Institute (12).
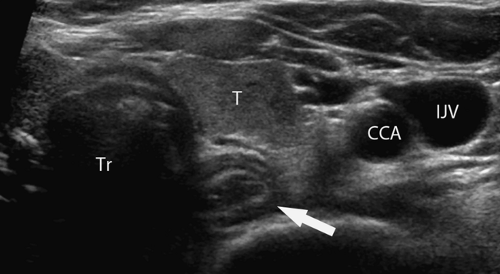
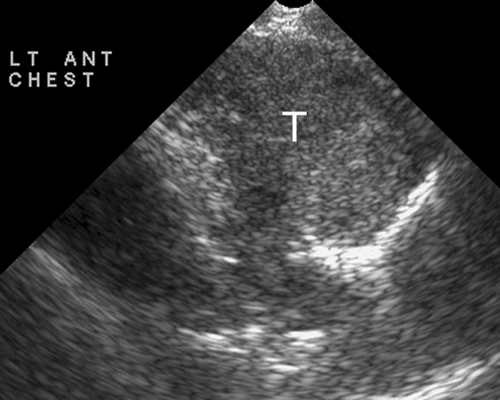 Figure 38.8. Mediastinal Mass. A left parasternal US image shows a large solid mediastinal mass (T). US-guided fine-needle and core biopsy was easily performed and confirmed a malignant thymoma. |
US is used to precisely guide percutaneous FNA and core biopsy of thyroid nodules, to screen patients at high risk for thyroid cancer, to identify recurrent disease in patients with known thyroid cancer, and to determine if palpable nodules arise from the thyroid gland (13). CT and MR supplement US by staging of invasive thyroid cancers, evaluating for postoperative recurrence of thyroid cancer and demonstrating extension of goiter into the thorax. Radionuclide imaging, discussed in a subsequent chapter, evaluates the physiological function of the gland.
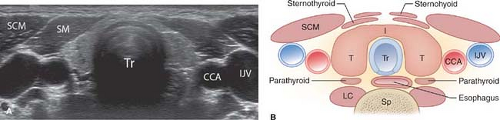
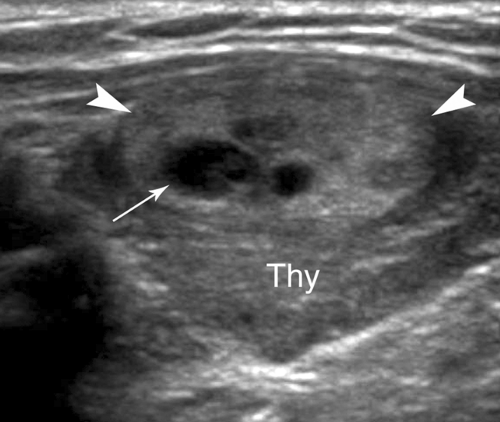
Normal US Examination and Anatomy. The thyroid gland consists of paired lobes of near-equal size (5 × 2 × 2 cm) connected across the trachea by a thin thyroid isthmus (Fig. 38.9). The thyroid parenchyma is homogeneous with fine medium-level echogenicity greater than that of the muscle. Anatomic landmarks include the midline air-filled trachea which casts an air shadow, the common carotid artery and internal jugular vein, which parallel the lateral edge of the thyroid lobes, the longus colli muscles posteriorly, and the sternohyoid, sternothyroid, and sternocleidomastoid muscles anteriorly. Small pools of colloid (colloid cysts) are routinely visualized within the normal gland. The thyroid lobes are often mildly asymmetric in size. The esophagus commonly protrudes from behind the trachea, nearly always on the left side, and must not be mistaken for a thyroid or parathyroid mass or lymph node (Fig. 38.10). The superior thyroid artery and vein are imaged between the upper pole of the thyroid and the longus colli. The recurrent laryngeal nerve and inferior thyroid artery and vein are seen posterior to the lower poles. The thyroid is easily imaged with the patient in a supine position with the neck extended by placement of a pillow beneath the shoulders (13). High-frequency (7 to 15 MHz) linear array transducers are used. The lobes of the thyroid gland are imaged and measured in longitudinal and transverse planes. The isthmus is imaged in the transverse plane and its thickness is recorded. The number, location, size in three dimensions, and characteristics of nodules are documented. The neck is examined for adenopathy or other abnormalities.
Thyroid Nodules
The Problem. Thyroid nodules are extremely common: 4% to 8% of adults have palpable nodules, 10% to 41% have nodules on US examination, and 50% have nodules at autopsy (11). Thyroid nodules increase in frequency with age and are much more common in women. Thyroid cancer, on the other hand, affects only 0.1% of the population. Thyroid cancer is less than 1% of all cancer and is the cause of less than 0.5% of all cancer deaths. Most thyroid cancers are slow growing and have low morbidity and mortality. The ratio of benign thyroid nodules to thyroid cancer can be estimated at as high as 500:1. The challenge of imaging studies and clinical evaluation is to establish the likelihood of malignancy and to select out for surgery only those patients with thyroid malignancy.
US is highly sensitive for the detection of thyroid nodules; however, its specificity for determining malignancy is low. Neither MR nor CT can improve that specificity. This is not surprising because the histological differentiation of benign follicular adenoma from well-differentiated follicular carcinoma is based solely on the identification of vascular invasion. Nodules considered suspicious for malignancy should undergo FNA biopsy for diagnosis. US-guided FNA of thyroid nodules is safe, accurate, and inexpensive (11). Complications, primarily hematoma and pain, are rare and minor.
Benign Thyroid Nodules
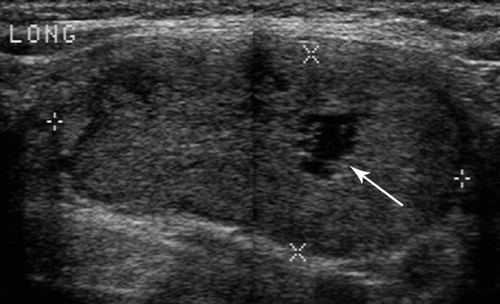
Adenomatous nodules, also called colloid nodules, are the most common thyroid nodule. They are not neoplasms but benign growths resulting from cycles of hyperplasia and involution of thyroid tissue. They are usually multiple and associated with diffuse enlargement of the thyroid gland. Individual nodules are isoechoic or hypoechoic to thyroid parenchyma and commonly show degenerative changes with prominent cystic components, necrosis, hemorrhage, and calcification (Fig. 38.11).
Follicular adenoma is the most common benign neoplasm. Autonomous hyperfunctioning adenomas are a cause of hyperthyroidism, but most adenomas cause no alteration of overall thyroid function. Most are solitary, solid, and well encapsulated (14). They may be hypoechoic, hyperechoic, or isoechoic to thyroid parenchyma (Fig. 38.12). Hyperfunctioning adenomas are commonly strikingly hypervascular on color flow US. Degenerative changes include focal necrosis, hemorrhage, edema, infarction, fibrosis, and calcification. Differentiation from follicular carcinoma is difficult; therefore an FNA cytologic diagnosis of follicular neoplasm is commonly considered an indication for surgical removal and histologic determination of the presence of cancer.
Thyroid cysts are extremely rare, epithelial-lined, simple cysts. Most cystic nodules found in the thyroid are actually cystic degeneration of an adenomatous nodule (“colloid cyst”) (Fig. 38.13) or a follicular adenoma.
Hemorrhage may occur into an adenomatous nodule or a follicular adenoma, or spontaneously into normal parenchyma. Patients present with sudden neck pain and subsequent swelling. US reveals a hypoechoic nodule with internal debris.
Malignant Thyroid Nodules
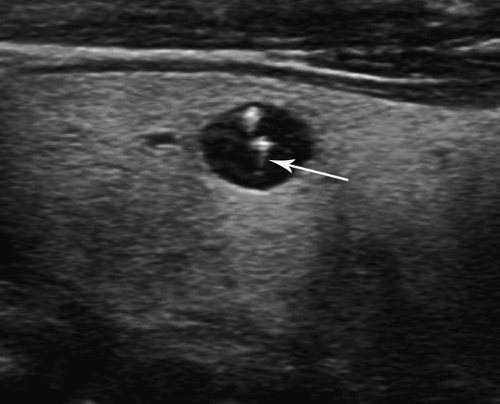
Papillary thyroid carcinoma (75% to 80% of thyroid cancer) is one of the least aggressive cancers in humans (15). Most patients are female (4:1). Nodules are hypoechoic and commonly multiple. Punctate internal calcifications (Fig. 38.14), representing psammoma bodies, are common (42%) and highly indicative of malignancy. Some tumors show the characteristic microcalcifications in the thyroid parenchyma without a discrete mass present. Involved cervical nodes may contain similar calcifications. The tumor spreads commonly to regional nodes, but rarely (2% to 3%) spreads to lung or bone. Five-year survival is 95% to 99%.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree