This article focuses on the current role of magnetic resonance imaging in the detection and characterization of chronic hepatitis and cirrhosis. In particular, the characteristic MR imaging features of morphologic changes and focal manifestations of chronic liver disease are highlighted.
Chronic liver diseases represent a major cause of morbidity and mortality worldwide. The major origins of chronic liver disease and also leading causes of cirrhosis and hepatocellular carcinoma (HCC) are chronic infection with hepatitis B virus (HBV) and hepatitis C virus (HCV), and alcoholic and nonalcoholic fatty liver disease. Magnetic resonance (MR) imaging has been increasingly used to evaluate diffuse parenchymal abnormalities of the liver. Morphologic changes and signal intensity effects not only facilitate the diagnosis of chronic liver disease with MR imaging, but may help to distinguish between differing etiology and assist in staging severity. Moreover, recent advances in the development of MR systems and liver-specific MR contrast agents such as superparamagnetic iron oxide (SPIO) and gadoxetic acid (Gd-EOB-DTPA) have expanded the potential utility of MR imaging in the accurate depiction of specific disorders and cirrhosis-associated hepatocellular nodules.
In this article, the authors focus on the current role of MR imaging in the detection and characterization of chronic hepatitis and cirrhosis. In particular, the characteristic MR imaging features of morphologic changes and focal manifestations of chronic liver disease are highlighted.
Definition, etiology, and prevalence
Chronic hepatitis is defined as a continuous or recurrent inflammation of the liver for more than 6 months, with histologic changes of chronic liver damage. Pathologically it is characterized by lymphocytic infiltration, liver cell injury, necrosis, and fibrosis. Chronic hepatitis progresses from mild inflammation, to more severe inflammation and fibrosis, and eventually to cirrhosis. Cirrhosis is characterized by the replacement of liver tissue by fibrosis, scar tissue, and regenerative nodules, leading to the deterioration of liver function.
The most common causes of cirrhosis in the United States are HBV and HCV infection, either singly or combined, and alcohol abuse. Other causes of cirrhosis include nonalcoholic fatty liver disease, hemochromatosis, autoimmune disease, Wilson disease, primary sclerosing cholangitis, and primary biliary cirrhosis.
The clinical importance of chronic liver disease is reflected in the large numbers of affected patients and the frequency of the associated serious complications. Approximately 400 million people are chronically infected with HBV worldwide, of whom 25% to 40% die of cirrhosis and its end-stage complications. Chronic hepatitis C further affects approximately 200 million people with a greater prevalence in Western countries. The development of cirrhosis is common in chronic HCV, and the risk of HCC is 3% to 4% per year. Once chronic HCV infection is established, cirrhosis develops within 10 to 20 years in approximately 20% of patients. An estimated 10,000 deaths annually have been attributed to HCV-related diseases, and it is suggested that HCV may be responsible for nearly half of all HCC cases.
Alcoholic liver disease is among the most important causes of morbidity and mortality in the United States, accounting for up to 12,000 deaths each year, and representing more than 50% of liver disease–related deaths. Nonalcoholic fatty liver disease (NAFLD) is now recognized as an important clinical entity, affecting approximately 20% to 30% of the adult population in the Western world. NAFLD represents a disease spectrum ranging from isolated steatosis to more advanced disease with necroinflammatory change and fibrosis (nonalcoholic steatohepatitis or NASH), to cirrhosis in its most severe form. The prevalence of obesity and NAFLD in the United States translates into a substantial clinical problem, with more than 19% of obese individuals and 2% to 3% of the general population presenting with NASH.
MR imaging features
Morphologic and Signal Intensity Changes
Hepatitis is associated with infiltration of inflammatory cells in to the liver, which results in liver cell injury and edema. Such liver changes may be visualized as periportal edema, which is characterized by high signal intensity bands paralleling the portal vessels on T2-weighted images ( Fig. 1 ). Periportal edema is a common but nonspecific imaging finding in patients with severe acute hepatitis and is also described in chronic viral hepatitis. A similar appearance may also be seen in patients with malignant lymphadenopathy in the porta hepatis, biliary obstruction, cirrhosis, hepatic trauma, or transplant rejection.

As cirrhosis progresses from early to advanced or end stage, it gives rise to several intra- and extrahepatic changes, including regional morphologic changes in the liver, nodularity of the liver surface, splenomegaly, regenerative nodules, iron and fat deposition, and ascites, and the development of varices and collaterals. Although the classically described findings of cirrhosis are common in advanced cirrhosis, they are seen less frequently in the early stage of the disease, at which time the liver may appear normal on cross-sectional imaging, occasionally hampering imaging-based diagnosis.
Enlargement of the hilar periportal space ( Fig. 2 ) on MR imaging has been shown to be a useful sign in the diagnosis of early cirrhosis. It has been reported that this sign is visible in 98% of patients with early cirrhosis who do not have conventional signs (ie, splenomegaly, portosystemic collateral vessels, ascites, or surface nodularity), whereas this sign is seen in only 11% of patients with normal livers. Often, expansion of the major interlobar fissure (see Fig. 2 ) is seen in these patients with early cirrhosis. These findings are attributed to atrophy of the medial segment of the left hepatic lobe, suggesting that medial segment atrophy may be an initial morphologic change in early cirrhosis.

Hepatic morphologic changes typically seen in advanced cirrhosis include hypertrophy of the caudate lobe and lateral segments of the left lobe, and atrophy of both posterior segments of the right lobe and the medial segment of the left lobe. Other morphologic changes with high specificity for a diagnosis of cirrhosis include the expanded gallbladder fossa sign ( Fig. 3 ), which is defined as enlargement of the pericholecystic space (ie, gallbladder fossa), and the right posterior hepatic notch sign (see Fig. 3 ), which is defined as a sharp indentation in the right medial posterior surface of the liver ( Table 1 ).

| Early Cirrhosis | Advanced Cirrhosis |
|---|---|
| Enlargement of the hilar periportal space | Hypertrophy of caudate lobe and/or lateral segments |
| Expansion of the major interlobar fissure | Atrophy of medial and/or posterior segments |
| Enlargement of the pericholecystic space (expanded gallbladder fossa sign) | |
| Hepatic sharp indentation in the posterior surface (right posterior hepatic notch sign) |
The patterns of hepatic morphologic and signal intensity changes overlap among the different causes of cirrhosis. However, certain imaging features may suggest particular etiological factors, such as enlargement of the lateral segment accompanied by shrinkage of both the right lobe and left medial segment, which reportedly frequently occurs in patients with viral-induced cirrhosis. Conversely, previous study showed that the mean values of the volume index of the caudate lobe were significantly greater in patients with alcoholic cirrhosis than in patients with viral cirrhosis. Marked caudate lobe enlargement is typically associated with alcoholic cirrhosis.
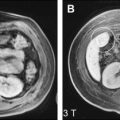
Primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC) have several distinctive features that may help to differentiate them from other types of cirrhosis ( Table 2 ). PSC is reportedly associated with hypertrophy of the caudate lobe and atrophy of the other areas (medial segment, lateral segment, and right hepatic lobe, either individually or in combination) ( Fig. 4 ). Previous study showed that these findings were observed in 68% (ie, hypertrophy of the caudate lobe) and 55% (ie, atrophy of the other areas) of patients with PSC, respectively. Other etiologies, such as Budd-Chiari syndrome also demonstrate hypertrophy of the caudate lobe and variable atrophy/hypertrophy of the remaining portions of the liver. In addition, irregular intra- and/or extrahepatic bile duct dilatation and stenosis are also observed. The arterial or delayed phase of contrast-enhanced dynamic MR imaging demonstrates increased enhancement of the hepatic parenchyma surrounding the dilated intrahepatic bile duct ( Fig. 5 ), which is considered to represent fibrotic changes and hepatocyte damage. In the authors’ experience, the periductal parenchyma in PSC does not show uptake of SPIO, a liver-specific MR contrast agent normally taken up by hepatic Kupffer cells ( Fig. 6 ). A reduced uptake of hepatobiliary-specific contrast agents (ie, Gd-EOB-DTPA, discussed later) is also observed (see Fig. 6 ).
| Morphologic Changes | Appearance on MR Imaging | |
|---|---|---|
| PSC | Hypertrophy of caudate lobe Atrophy of medial segment, lateral segment, right hepatic lobe (or all of 3 segments) Dilatation and stenosis of intra- and/or extrahepatic bile duct | Dynamic MR imaging a Increased enhancement of local hepatic parenchyma SPIO b Decreased enhancement of local hepatic parenchyma Gd-EOB-DTPA c Decreased enhancement of local hepatic parenchyma |
| PBC | Nonspecific | Periportal hyperintensity (hyperintense on T2WI) |
| MR imaging periportal halo sign (hypointense on T1WI and T2WI) |
a T1-weighted GRE image on arterial- and portal phase after administration of gadolinium-based contrast agents.
b T2-weighted GRE image after administration of superparamagnetic iron oxide (SPIO).
c T1-weighted GRE image on hepatocyte-selective phase after administration of gadoxetic acid (Gd-EOB-DTPA).



MR findings that have been shown to be helpful in the diagnosis of PBC include periportal hyperintensity on T2-weighted images and the periportal halo sign ( Fig. 7 ). Periportal hyperintensity on T2-weighted MR images has been attributed to periportal inflammation. One study reported that periportal hyperintensity (see Fig. 7 ) was observed in 100% of patients with PBC with histologic stage I or II disease, 75% of patients with stage III disease, and 33% of patients with stage IV disease. Forty-three percent of patients with PBC are reported to demonstrate the periportal halo sign (see Fig. 7 ), which is depicted as periportal signal hypointensity on T1- and T2-weighted MR images. It has been suggested that the MR imaging periportal halo sign may represent stellate, periportal, hepatocellular parenchymal extinction encircled by a rosette of large regenerating nodules.

Portal Hypertension
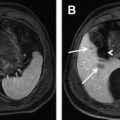
In the cirrhotic liver, progressive hepatic fibrosis leads to increased vascular resistance at the level of the hepatic sinusoids, which results in a reduced portal contribution to liver perfusion. The subsequent development of portal hypertension gives rise to complications such as ascites and the development of collateral vessels at the lower end of the esophagus ( Fig. 8 ). Portosystemic shunts also form through reopened paraumbilical veins (see Fig. 8 ) and the left gastric vein, which both normally drain into the portal vein.

The decreased portal venous supply that occurs as a result of liver fibrosis is partially compensated by an increase in arterial blood supply. Such an increase in arterial perfusion may be demonstrated by pronounced liver enhancement in the first seconds after administration of intravenous contrast media in cirrhotic patients. Early patchy enhancement of liver parenchyma on MR imaging is a feature of portal hypertension, and is reportedly associated with the presence of numerous infiltrating macrophages, necrosis, tissue collapse, and increased steatosis ( Fig. 9 ).
Fibrosis
Pathologic characteristics and MR imaging features of liver fibrosis
Fibrosis is an inherent component of cirrhosis, and has MR imaging characteristics. In viral hepatitis, liver fibrosis begins and manifests as fibrous expansion of the portal triads ( Fig. 10 ). Fibrous septa then grow from the expanded portal triad into the surrounding hepatic parenchyma. Subsequently, the fibrous septa lengthen and thicken to eventually form fibrous bridges that link adjacent portal triads and central veins (see Fig. 10 ). As the liver injury continues, the bridges continue to enlarge and coalesce and eventually divide the liver into rounded islands of hepatic parenchyma (regenerative nodules) surrounded by fibrosis tissue (see Fig. 10 ). The pattern of early fibrosis differs in alcoholic hepatitis and nonalcoholic fatty liver disease because early fibrosis first develops adjacent to the central veins rather than in the portal triads, but progressive fibrosis eventually shows the same pathologic findings as cirrhosis caused by viral hepatitis.
The current reference examination in the assessment of liver fibrosis is liver biopsy. However, this procedure is invasive with recognized morbidity and mortality, and repeated biopsy for the monitoring of disease progression is accordingly suboptimal. In addition, the accuracy of biopsy remains controversial because of sampling variability caused by the small size of hepatic samples and the heterogeneity of liver fibrosis. These limitations have stimulated the search for noninvasive approaches to the assessment of liver fibrosis.
Conventional MR imaging techniques and fibrosis
The MR imaging appearance of the fibrotic septa and bridges comprises reticulations surrounding regenerative nodules giving rise to the so-called lacelike pattern. The fibrous septa appear hypointense on T1-weighted images and hyperintense on T2-weighted images ( Fig. 11 ), which in part is attributed to large water content.
Most gadolinium-based contrast agent formulations freely equilibrate with extracellular volumes, such as liver fibrosis, and thereby improve the visibility of fibrosis on MR imaging. MR images obtained at the equilibrium and delayed phase after gadolinium administration show fibrotic septa and bridges as linear and reticulation enhancement patterns. These findings are more prominent in the periphery of the liver.
In end-stage liver cirrhosis, focal confluent fibrosis, which typically has a wedge shape or geographic shape with straight or concave borders, is occasionally observed in the subcapsular region. The signal intensity and enhancement features of confluent fibrosis following administration of extracellular contrast agents are similar to those of fibrotic septa and bridges.
Liver-specific contrast agents and fibrosis
SPIO-enhanced MR imaging has been shown to be helpful in the detection of macroscopic fibrous bands and diffuse liver fibrosis. On T2-weighted turbo spin-echo and T2-weighted GRE images after administration of SPIO, the areas of fibrosis within the liver, which have reduced Kupffer cell density, accumulate less iron oxide and appear as hyperintense reticulations or areas (ie, confluent fibrosis) compared with the surrounding hepatic parenchyma ( Fig. 12 ). A recent study has shown the usefulness of double-contrast–enhanced MR imaging (sequential administration of SPIO and a gadolinium-based contrast agent) in the detection of liver fibrosis architecture. The investigators noted that the combination of these contrast agents was synergistic, and demonstrated liver fibrosis with greater clarity than could be achieved with either agent alone.
Hepatobiliary-specific contrast agents such as mangafodipir trisodium (Mn-DPDP), gadobenate dimeglumine (Gd-BOPTA), and Gd-EOB-DTPA are taken up by functioning hepatocytes and excreted in the bile. The paramagnetic properties of these agents cause shortening of the longitudinal relaxation time (T1) of the liver and biliary tree. In visual analysis of images enhanced by Mn-DPDP, lower or heterogeneous enhancement areas are observed in cirrhotic liver, and it is considered that these areas contain the fibrous zone, indicating reticulation, confluent, and hepatocyte necrosis. In the authors’ experience, images enhanced by Gd-EOB-DTPA frequently show similar findings in cirrhotic liver, and this agent may also distinctly demonstrate liver fibrosis, such as fibrous septa, bridges ( Fig. 13 ), and confluent fibrosis (see Fig. 13 ) ( Table 3 ).
| Macroscopic Findings | MR Imaging Form | Signal Intensity | Dynamic MR Imaging a | SPIO b | Gd-EOB-DTPA c |
|---|---|---|---|---|---|
| Fibrotic septa and bridge | Reticulation (lacelike pattern) | Hypointense (T1WI) | Increased enhancement | Hyperintense d | Hypointense d |
| Confluent fibrosis | Wedge shape or geographic shape | Hyperintense (T2WI) |
a T1-weighted GRE image on delayed phase after administration of gadolinium-based contrast agents.
b T2-weighted GRE image after administration of SPIO.
c T1-weighted GRE image on hepatocyte-selective phase after administration of Gd-EOB-DTPA.
d Appearance is described in comparison with the surrounding hepatic parenchyma.
Emerging functional MR imaging techniques for fibrosis
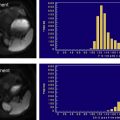
Recently, several novel techniques for the assessment of liver fibrosis have been proposed, including MR elastography, diffusion-weighted MR imaging, and MR spectroscopy. MR elastography is a phase contrast-based MR imaging technique for direct visualization and quantitative measurement of propagating mechanical shear waves in biologic tissue. Recent studies in patients with a spectrum of liver disease types have shown that liver stiffness as measured with MR elastography increases as the stage of fibrosis advances. The difference in stiffness between patients with early stages of fibrosis (F0 vs F1 vs F2) are small, with overlap between groups, but those between groups at higher stages (F2 vs F3 vs F4) are large, with little overlap. Evaluation of the reproducibility and validity of MR elastography in an independent population of 35 healthy individuals and 48 patients with varying degrees of chronic liver disease showed a sensitivity of 86% and specificity of 85% for the detection of stages 2 to 4 fibrosis compared with liver histology from biopsy. A high negative predictive value (97%) for excluding the presence of fibrosis was also noted, suggesting that MR elastography might have a role in improving the ability to risk-stratify patients for liver biopsy to exclude occult advanced fibrosis. MR elastography therefore appears to shows promise for the noninvasive staging of liver fibrosis, particularly in patients with advanced fibrosis.
Diffusion-weighted magnetic resonance imaging is a technique that assesses the freedom of diffusion of water protons within tissue by applying motion-sensitizing gradients that cause diffusing protons to lose signal. Recent advances in MR imaging technology have facilitated the performance of diffusion-weighted MR imaging of the liver, and it has also been used to detect liver fibrosis. Prior studies have reported that apparent diffusion coefficient (ADC) values acquired from b values of 500 (seconds/mm 2 ) and greater correlated significantly with liver fibrosis stage, and that ADC values with a combination of b value of 0 and 1000 (seconds/mm 2 ) showed the highest correlation (r = −0.654, P <.001). On the other hand, several studies noted that there was no significant correlation between fibrosis stage and the ADC value using low b values (b values, 50 to 400 seconds/mm 2 ), because diffusion-weighted imaging with a low b value was influenced by perfusion contamination. Luciani and colleagues, reported that ADC calculated from low b values was significantly reduced in cirrhosis. Thus, the fast component diffusion-weighted MR imaging obtained with low b values may provide information related to microperfusion changes in diffuse liver disease whereas the slow component diffusion-weighted MR imaging obtained with high b values has been suggested to reflect a decrease in water proton diffusion. The principles of diffusion-weighted MR imaging is discussed further in this presentation on functional MR imaging techniques.
In vivo MR spectroscopy (MRS) is most commonly used to assess signals from hydrogen ( 1 H) and phosphorus ( 31 P). Although 1 H-based MRS allows for the quantification of certain metabolites and lipids, 31 P-based MRS provides insights on processes, including cell turnover and energy state, based on the substantial 31 P concentrations within hepatocytes. Previous studies have suggested MRS may be useful in detecting hepatic fibrosis. An increased levels of hepatic phosphomonoesters (PME) have been reported in patients with established cirrhosis, and an increasing PME to phosphodiester (PDE) ratio has been reported to correlate with worsening necroinflammatory and fibrosis scores on liver histology. It has also been suggested that a PME and PDE ratio 0.2 or less is correlated with mild hepatitis and 0.3 or greater is correlated with cirrhosis in a study involving patients with chronic hepatitis C. Despite some preliminary promising data, 31 P-based MRS is not widely used due to specific technical requirements. The role of MRS in the detection of liver inflammation and fibrosis requires further investigation.
MR imaging features
Morphologic and Signal Intensity Changes
Hepatitis is associated with infiltration of inflammatory cells in to the liver, which results in liver cell injury and edema. Such liver changes may be visualized as periportal edema, which is characterized by high signal intensity bands paralleling the portal vessels on T2-weighted images ( Fig. 1 ). Periportal edema is a common but nonspecific imaging finding in patients with severe acute hepatitis and is also described in chronic viral hepatitis. A similar appearance may also be seen in patients with malignant lymphadenopathy in the porta hepatis, biliary obstruction, cirrhosis, hepatic trauma, or transplant rejection.