The liver is one of the most challenging organs of the body to image with magnetic resonance because it is large and mobile, receives a dual blood supply, and is surrounded by organs and structures that contribute to artifacts from flow and susceptibility. Recent advances in imaging hardware, in addition to improvements in temporal resolution and development of hepatocyte-specific contrast agents, make imaging of the liver more approachable than in the past; however, it remains a complex process that requires compromise. In this article the authors discuss development and optimization of a liver imaging protocol at 1.5 T, with common variations in each element of the protocol, as well as the strengths and weaknesses associated with the relevant sequences.
Introduction and challenges of liver MR imaging
The liver is one of the most challenging organs of the body to image with magnetic resonance (MR). Much of the liver’s posterior, lateral, and superior margins are subject to lung-related susceptibility effects, while bowel skirts its inferior and anterior edges. The left hemiliver lies just under the constantly moving heart, a liability for such motion-sensitive techniques as diffusion-weighted imaging (DWI). Pulsatile flow within the aorta and respiratory motion of the gallbladder can create phase errors that simulate disease. The dual hepatic blood supply and variability in tumor vascularity demand precise timing of multiphase contrast-enhanced acquisitions. These challenges are further compounded by the fact that the liver is one of the largest and most mobile organs of the abdomen. As a result, liver MR imaging requires many compromises, such as between signal-to-noise ratio (SNR), spatial and temporal resolution, artifact suppression, and lesion conspicuity.
In recent years, clever hardware and pulse sequence innovations and the introduction of new liver-specific contrast agents together have helped mitigate these many obstacles to push MR imaging to the forefront of liver imaging. Nonetheless, liver MR imaging is far from standardized. The plethora of available vendors, systems, coils, sequences, and imaging parameters that have made liver MR imaging so successful have also made it dauntingly complex. In fact, the title of this article is a misnomer. There is in essence no optimal union of imaging parameters, only a marriage of convenience subject to the laws of physics and the constraints of one’s equipment, practice environment, and economics. In this article, the authors provide guidance regarding the development of a liver MR imaging protocol. For each protocol component (eg, T2-weighted sequence), the many available variations, with their strengths and weaknesses, are discussed. Along the way, the recognition and elimination of imaging pitfalls and artifacts frequently encountered during liver imaging with MR are also discussed. For simplicity, this discourse will primarily focus on imaging at a field strength of 1.5 Tesla (T), as a dedicated discussion of imaging the liver at 3.0 T can be found elsewhere in this issue.
Basic principles of sequence optimization
Obtaining images with MR requires both homogeneous static magnetic fields and transmitted radiofrequency (RF) fields, rapid and accurate spatial encoding, and clear signal reception. Static field (B 0 ) homogeneity is a function of both magnet design and patient anatomy, and is shimmed through active and passive means. The uniformity of the transmitted RF field (B 1 ) is a function of field strength, coil design, the transmitted RF pulse, and patient body habitus. B 1 inhomogeneities become more problematic at higher field strengths, in larger patients, and in the presence of ascites. Such B 1 inhomogeneities contribute to spatial variations in SNR and flip angle that can have an adverse effect on image contrast and uniformity of fat suppression. A variety of strategies have been developed to reduce the effects of B 1 nonuniformity, including the use of dielectric pads, RF shimming, and adiabatic pulses. Because these issues are more relevant to a discussion of 3.0-T imaging, they are not discussed further here.
Clear signal reception requires use of an appropriate receiver coil. The development of dedicated receiver coils directly applied to the surface of the patient is considered a major advance in body MR imaging. Coils applied directly over the patient, conforming to the curvature of the body wall, dramatically improve SNR by bringing the receiver elements closer to the signal source ( Fig. 1 ). The use of multicoil, multichannel arrays and quadrature coil geometry can further the potential gain in SNR, while improving efficiency by permitting applications such as parallel imaging. Such is the improvement in image quality that the use of a surface coil is considered mandatory, when possible, for the performance of high-quality liver MR imaging.

Once a suitable environment for exciting protons and receiving signal has been established, imaging parameters are manipulated and compromises made between 5 main factors:
- 1.
Signal-to-noise ratio (SNR)
- 2.
Contrast-to-noise ratio (CNR)
- 3.
Spatial resolution
- 4.
Temporal resolution
- 5.
Artifacts.
Signal-to-Noise Ratio
SNR is the strength of the signal relative to the noise inherent in the system. Signal is a function of the net transverse magnetization in the body as each echo is collected. Increases in B 0 field strength and voxel volume improve SNR, although the latter compromises spatial resolution. Voxel size can be increased in the through-plane (slice or partition thickness) or in-plane (matrix) axes, with the benefit of reducing acquisition time if all other parameters are kept constant. A reduction in receiver bandwidth improves SNR at the expense of temporal resolution (achievable echo and repetition times) and increased chemical shift artifact. For example, a typical 3-dimensional (3D) fat-suppressed T1-weighted gradient echo sequence used for liver imaging might use the following parameters:
Marix = 320 × 160, Partition thickness 5 mm overlapping 50%, Flip angle 12°, Bandwidth + 62.5 kHz, Acceleration factor = 2
Total acquisition time to cover the liver with this sequence would be approximately 23 seconds. The acquisition time could be reduced to 13 seconds by reducing the matrix to 256 × 128, increasing the partition thickness from 5 mm to 6 mm, and increasing the receiver bandwidth to +100 kHz without affecting SNR (the loss of SNR caused by the increased bandwidth is compensated for by the gain in SNR caused by the decreased matrix and increased partition thickness).
The number of signal averages also affects SNR, and can be thought of as the amount of data stored in each line of k-space. Doubling the number of signal averages increases the signal by the square root of 2, but with the expense of doubling the imaging time. Imaging parameters repetition time (TR), echo time (TE), and flip angle also influence signal but are generally considered more important determinants of image contrast. While high SNR is desirable, it is not the ultimate goal of liver imaging. Rather, an abundance of signals facilitates additional strategies to improve spatial and temporal resolution.
Contrast-to-Noise Ratio
CNR is a far more critical measure than SNR of the clinical utility of an image. CNR is the difference in signal intensity between 2 different regions of interest in the same image, scaled to noise. CNR is a measure of lesion conspicuity and is affected, to an extent, by the same factors that influence SNR; however, because CNR determines how visible an abnormality will appear on an image, parameters affecting image contrast can have a profound influence on CNR. For example, increases in flip angle for a constant TR can reduce image SNR by preventing full recovery of tissue magnetization between excitation pulses. However, such a strategy can improve the CNR between enhancing vessels and background liver (to a point) after administration of gadolinium-based contrast material. Increasing the TE will improve liver lesion contrast for cysts, hemangiomas, bile, and other tissues and substances with long T2 relaxation times at the expense of SNR.
Spatial Resolution
Spatial resolution, the factor that controls detail discrimination, is primarily controlled by voxel size. In general, high-resolution images of the liver are desirable to resolve small focal lesions and anatomic structures; however, improvements in spatial resolution typically come with compromises in temporal resolution or SNR. When optimizing spatial resolution, one must determine the minimum acceptable SNR and maximum tolerable breath-hold duration for a sequence. As a general rule, lower spatial resolution is preferable to severe respiratory motion artifact, as the high contrast sensitivity inherent to MR imaging can compensate for larger voxel size. The use of respiratory triggering permits high-resolution imaging without compromising SNR but is not practical for imaging discrete vascular phases after intravenous contrast administration. An apparent increase in spatial resolution without significant time penalty can be achieved with zero fill interpolation, a technique that fills portions of k-space with zeros to interpolate the acquired matrix to one of higher apparent resolution. In-plane or through-plane zero filling can be applied, and this technique is often employed in the fat-suppressed 3D sequences used for dynamic contrast-enhanced imaging.
Temporal Resolution
Optimizing temporal resolution is critical to achieving motion-free breath-hold imaging of the liver, particularly during the dynamic phases of contrast enhancement. Dynamic contrast-enhanced liver imaging is typically performed with a 3D fat-suppressed T1-weighted gradient echo sequence. In most cases, the TR is kept as short as possible, with T1 contrast for this short TR maintained by use of a reduced flip angle. With the exception of newer dual-echo techniques, many manufacturers use an opposed-phase echo time for their dynamic liver sequence, leaving little room for further reduction of the TE at 1.5 T. Despite being reasonably optimized for breath-hold imaging, further improvements in temporal resolution can be gained over the “out-of-the-box” configuration, albeit at a price.
The simplest strategy to reduce scan time is to lower spatial resolution. Aside from potentially obscuring small structures and abnormalities, decreasing resolution can exacerbate truncation artifact (edge ringing that propagates through the images in the phase-encoding direction). The use of zero filling can help mitigate some of the effects of lower imaging matrices but can also exacerbate truncation artifact. Fortunately, truncation artifacts are rarely of clinical significance when imaging the liver using currently available pulse sequences and typical matrices (256 × 128 or higher). The SNR gained by lower spatial resolution can offset SNR lost by increasing the receiver bandwidth to further reduce acquisition time. Additional time savings can be achieved through the use of a reduced-phase field of view and partial Fourier methods.
Parallel Imaging
Parallel imaging has become a mainstay of hepatic MR imaging, allowing dramatic improvements in temporal resolution with a few trade-offs. These techniques save time by decreasing the number of individual phase-encoding steps necessary to create a complete image, which is accomplished by using the spatial information from individual coil elements in tandem with conventional gradient-based spatial encoding. These sequences are known as sensitivity encoding (SENSE), modified SENSE (mSENSE), or generalized autocalibrating partially parallel acquisition (GRAPPA), depending on the vendor and the method of data manipulation, either within k-space (GRAPPA) or within image space. In addition, these techniques vary with respect to calibration techniques. GRAPPA and mSENSE are autocalibrating, acquiring calibration data during image acquisition, whereas other techniques require dedicated calibration scans.
The use of parallel imaging can significantly reduce acquisition times, and is now considered routine for liver imaging. The reduction in scan time is related to the number of lines of k-space that are acquired in parallel during the phase encoding process (often referred to as the acceleration factor). This factor is higher for single-shot T2-weighted sequences (sometimes as high as 4). Signal, in this case, is maintained by the shorter echo train length. An acceleration factor of 2 or less is common for most abdominal applications, although higher factors are becoming more achievable on newer platforms. Some newer 3D sequences and coils allow for parallel imaging to be applied in the in-plane and through-plane direction simultaneously.
One downside of parallel imaging can be a reduction in SNR that worsens as the parallel imaging acceleration factor increases. This trend can be offset in many cases by adjustments in other parameters, although the signal gain through the use of gadolinium-based contrast media minimizes the impact of signal loss at typical acceleration factors of 2 or less. Another potential hazard associated with the use of parallel imaging is related to artifacts generated during image reconstruction. SENSE-based techniques reduce the number of phase encoding steps by acquiring data with a reduced phase field of view. The resulting aliased image is “unwrapped” using coil sensitivity profiles obtained during the calibration process. However, if the prescribed field of view is too small in the phase encoding direction, the unwrapping is incomplete and residual signal contaminates the central portion of the image. In addition, when there are too few phase-encoding steps acquired to maintain signal, or if the patient is large, noise amplification can be severe in the central portion of the image. When the field of view is increased in the phase direction in both cases, these artifacts can be reduced ( Fig. 2 ). Misregistration between the calibration scan and the image acquisition can also lead to artifacts ( Fig. 3 ), a common occurrence with breath-hold techniques when respiration is suspended inconsistently between the calibration scan and the image acquisition. Calibration artifacts can be corrected by recalibrating or by using one of the autocalibrating methods of parallel imaging. Table 1 provides an overview of the effects of changes in the some of the key parameters of sequence optimization.


| Parameter | Scan Time | Spatial Resolution | SNR | Artifact |
|---|---|---|---|---|
| Receiver bandwidth | An increase in bandwidth increases sampling rate and allows shorter TE, decreasing scan time | No direct effect on spatial resolution | SNR is decreased with increasing bandwidth | Water/fat misregistration is exacerbated at lower bandwidths |
| Acceleration factor (parallel imaging) | Increasing acceleration factor decreases scan time | No direct effect but may allow for improved spatial resolution for a given scan time | Increasing the parallel imaging factor decreases SNR | Artifacts related to aliasing and callibration are propagated in the phase-encoding direction |
| Field of view | Increasing field of view increases scan time if spatial resolution is kept constant | If the matrix is kept constant, voxel size will increase, resulting in decreased spatial resolution | If the matrix is kept constant, SNR increases due to larger voxel size | Most commonly associated with aliasing when the field of view is decreased and excludes anatomy. Artifact appears in the center of an image with parallel imaging if anatomy is excluded in phase-encoding direction |
| Matrix | Scan time is increased with a finer matrix in the phase-encoding axis | Spatial resolution is increased with an increase in matrix for constant field of view | SNR is decreased with smaller voxel size | Truncation artifact may be seen with marked reduction in matrix |
| Slice thickness | Increasing slice thickness decreases scan time for constant anatomic coverage | Through-plane resolution is decreased when slice thickness is increased | SNR increases with increasing slice thickness | Degradation of multiplanar reconstructions and worsened volume averaging effects with increased slice thickness |
| Number of signal averages (NEX, NSA) | Increasing NSA increases scan time | No effect on spatial resolution | SNR increases with increasing NSA | May introduce image blurring with higher NSA |
Additional Artifacts
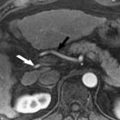
Parallel imaging is just one potential source of artifacts encountered during image acquisition and interpretation. One of the most common sources of artifacts encountered in liver imaging is motion-induced phase errors. Motion is problematic because it can introduce phase shifts that are not induced by the spatial encoding gradients, resulting in misplaced signal on the image. Motion occurring between phase-encoding steps or during application of the imaging gradients can result in “ghost” artifacts along the phase encoding axis. The two most common sources of ghost artifacts are respiratory motion ( Fig. 4 ) and vascular flow. The former source can be addressed with careful respiratory triggering, breath-hold imaging, fat suppression, or placement of saturation bands over high signal fat in the anterior abdominal wall. Vascular flow-related artifacts can be mitigated through the use of gradient moment nulling (also known as flow compensation) or saturation bands placed above and below the imaging volume. However, gradient moment nulling, as typically used, only corrects for phase errors induced by flow at constant velocity (ie, first order flow). Phase ghosts are problematic because they can mimic hepatic lesions, particularly within the left lateral section of the liver ( Fig. 5 ). The anatomic structures most likely to produce motion-related ghost artifacts in the liver include the anterior abdominal wall, gallbladder, inferior vena cava, and aorta.


Wraparound artifact occurs when objects or body parts outside the field of view are spatially mismapped to within the image. Wraparound artifact occurs because the image is only encoded for a maximum of 360° of phase shift. Any phase shift outside of this range (due to the object’s location outside the field of view) is misrepresented on the image. Protons experiencing higher degrees of phase shift, for example 365°, will appear to have experienced only 5° of shift, resulting in their being spatially mapped to the low-shift side of the image ( Fig. 6 ). Because 3D acquisitions use phase encoding to distinguish partitions in the slice direction, wraparound can occur in this axis as well as in the in-plane phase-encoding direction (typically anterior to posterior for a liver acquisition). Wraparound artifact can be eliminated or reduced by increasing the field of view, to move the offending structure out of the imaging region where wraparound occurs. or through the use of saturation bands to reduce signal in the source of the artifact. Alternatively, one can use an option known as phase oversampling or “no-phase-wrap” to sample outside the field of view in the phase encoding direction. However, this method is not preferred for breath-hold imaging, as it can increase acquisition time significantly. By swapping the phase and frequency directions, wraparound artifact can be shifted off of structures of interest, but this eliminates the time-saving benefit of using a rectangular field of view. The use of SENSE-based parallel imaging techniques can cause a distinctive wraparound artifact near the center of the image, as the image cannot be completely unwrapped if the field of view is too small (see Fig. 2 ).

Differing magnetic susceptibilities of tissues and substances within the abdomen is a common source of artifacts during liver imaging. Substances, such as air or metal, cause magnetic field inhomogeneities that result in local signal loss, geometric distortion, and failure of spectrally selective forms of fat suppression. In this manner, cholecystectomy clips can obscure the porta hepatis region, and gas within the colon can create the appearance of tumors within the adjacent liver ( Fig. 7 ). Gradient echo–based sequences are most sensitive to susceptibility differences, as the lack of RF refocusing pulses prevents correction for T2* decay. Susceptibility artifact can be reduced by minimizing TE as gas and ferromagnetic particles such as clips, wires, and ballistic fragments create local field inhomogeneities that become more pronounced over time. Shorter echo times may be achieved by using fractional echo sampling and higher receiver bandwidths.

The Difficult Patient
Some patients present unique challenges due to habitus, claustrophobia, or limited breath-holding capacity. The most important factor to be considered initially for all patients is comfort. A comfortable patient is more likely to remain still and comply with instructions. For patients with lung disease, supplemental oxygen can improve comfort and increase breath-holding compliance. Dramatic reductions in scan time can be achieved through simple sequence modifications such as increased slice or partition thickness, reduced matrix, and increased bandwidth. Because of the high contrast sensitivity of MR imaging, a low-resolution, motion-free image is often more clinically useful than a high-resolution image degraded by motion.
For dynamic enhanced imaging of the liver, diagnostic data sets covering the entire liver can be obtained in less than 10 seconds when necessary. For patients who cannot suspend respirations for more than a few seconds, acquiring data during shallow continuous breathing or with respiratory triggering might be an option. When respiratory triggering is not an option, motion-resistant sequences such as single-shot echo train spin echo, steady-state free precession (SSFP), and magnetization-prepared gradient echo can be considered. Protocols should be as efficient as possible to prevent patient fatigue, and critical breath-hold sequences should be accomplished as soon as possible in the imaging protocol.
Coil positioning and clearance within the magnet can be challenging in large patients. Normal landmarks for coil positioning may not be apparent to the technologist; therefore, scout imaging with special attention to coil placement relative to the area of interest is important. The technologist should not hesitate to reposition the surface coil when necessary. Failure to do so results in signal-starved areas at the coil margins. A patient with a protuberant abdomen may require additional padding under the free ends of the surface coil to prevent movement that can contribute to calibration errors when using parallel imaging. In those patients for whom coil placement is not possible because of insufficient magnet clearance, the intrinsic body coil can be used with sequence modifications to compensate for relative loss of signal. In all cases, excellent communication between the patient and technologist is essential to ensuring patient compliance and satisfactory image quality. Coaching the patient and attention to respiratory waveforms can improve acquisition timing, and communication with the patient helps keep the patient calm and focused on the examination. Light sedation can be helpful, but heavy sedation can hinder patient compliance.
Basic principles of sequence optimization
Obtaining images with MR requires both homogeneous static magnetic fields and transmitted radiofrequency (RF) fields, rapid and accurate spatial encoding, and clear signal reception. Static field (B 0 ) homogeneity is a function of both magnet design and patient anatomy, and is shimmed through active and passive means. The uniformity of the transmitted RF field (B 1 ) is a function of field strength, coil design, the transmitted RF pulse, and patient body habitus. B 1 inhomogeneities become more problematic at higher field strengths, in larger patients, and in the presence of ascites. Such B 1 inhomogeneities contribute to spatial variations in SNR and flip angle that can have an adverse effect on image contrast and uniformity of fat suppression. A variety of strategies have been developed to reduce the effects of B 1 nonuniformity, including the use of dielectric pads, RF shimming, and adiabatic pulses. Because these issues are more relevant to a discussion of 3.0-T imaging, they are not discussed further here.
Clear signal reception requires use of an appropriate receiver coil. The development of dedicated receiver coils directly applied to the surface of the patient is considered a major advance in body MR imaging. Coils applied directly over the patient, conforming to the curvature of the body wall, dramatically improve SNR by bringing the receiver elements closer to the signal source ( Fig. 1 ). The use of multicoil, multichannel arrays and quadrature coil geometry can further the potential gain in SNR, while improving efficiency by permitting applications such as parallel imaging. Such is the improvement in image quality that the use of a surface coil is considered mandatory, when possible, for the performance of high-quality liver MR imaging.
Once a suitable environment for exciting protons and receiving signal has been established, imaging parameters are manipulated and compromises made between 5 main factors:
- 1.
Signal-to-noise ratio (SNR)
- 2.
Contrast-to-noise ratio (CNR)
- 3.
Spatial resolution
- 4.
Temporal resolution
- 5.
Artifacts.
Signal-to-Noise Ratio
SNR is the strength of the signal relative to the noise inherent in the system. Signal is a function of the net transverse magnetization in the body as each echo is collected. Increases in B 0 field strength and voxel volume improve SNR, although the latter compromises spatial resolution. Voxel size can be increased in the through-plane (slice or partition thickness) or in-plane (matrix) axes, with the benefit of reducing acquisition time if all other parameters are kept constant. A reduction in receiver bandwidth improves SNR at the expense of temporal resolution (achievable echo and repetition times) and increased chemical shift artifact. For example, a typical 3-dimensional (3D) fat-suppressed T1-weighted gradient echo sequence used for liver imaging might use the following parameters:
Marix = 320 × 160, Partition thickness 5 mm overlapping 50%, Flip angle 12°, Bandwidth + 62.5 kHz, Acceleration factor = 2
Total acquisition time to cover the liver with this sequence would be approximately 23 seconds. The acquisition time could be reduced to 13 seconds by reducing the matrix to 256 × 128, increasing the partition thickness from 5 mm to 6 mm, and increasing the receiver bandwidth to +100 kHz without affecting SNR (the loss of SNR caused by the increased bandwidth is compensated for by the gain in SNR caused by the decreased matrix and increased partition thickness).
The number of signal averages also affects SNR, and can be thought of as the amount of data stored in each line of k-space. Doubling the number of signal averages increases the signal by the square root of 2, but with the expense of doubling the imaging time. Imaging parameters repetition time (TR), echo time (TE), and flip angle also influence signal but are generally considered more important determinants of image contrast. While high SNR is desirable, it is not the ultimate goal of liver imaging. Rather, an abundance of signals facilitates additional strategies to improve spatial and temporal resolution.
Contrast-to-Noise Ratio
CNR is a far more critical measure than SNR of the clinical utility of an image. CNR is the difference in signal intensity between 2 different regions of interest in the same image, scaled to noise. CNR is a measure of lesion conspicuity and is affected, to an extent, by the same factors that influence SNR; however, because CNR determines how visible an abnormality will appear on an image, parameters affecting image contrast can have a profound influence on CNR. For example, increases in flip angle for a constant TR can reduce image SNR by preventing full recovery of tissue magnetization between excitation pulses. However, such a strategy can improve the CNR between enhancing vessels and background liver (to a point) after administration of gadolinium-based contrast material. Increasing the TE will improve liver lesion contrast for cysts, hemangiomas, bile, and other tissues and substances with long T2 relaxation times at the expense of SNR.
Spatial Resolution
Spatial resolution, the factor that controls detail discrimination, is primarily controlled by voxel size. In general, high-resolution images of the liver are desirable to resolve small focal lesions and anatomic structures; however, improvements in spatial resolution typically come with compromises in temporal resolution or SNR. When optimizing spatial resolution, one must determine the minimum acceptable SNR and maximum tolerable breath-hold duration for a sequence. As a general rule, lower spatial resolution is preferable to severe respiratory motion artifact, as the high contrast sensitivity inherent to MR imaging can compensate for larger voxel size. The use of respiratory triggering permits high-resolution imaging without compromising SNR but is not practical for imaging discrete vascular phases after intravenous contrast administration. An apparent increase in spatial resolution without significant time penalty can be achieved with zero fill interpolation, a technique that fills portions of k-space with zeros to interpolate the acquired matrix to one of higher apparent resolution. In-plane or through-plane zero filling can be applied, and this technique is often employed in the fat-suppressed 3D sequences used for dynamic contrast-enhanced imaging.
Temporal Resolution
Optimizing temporal resolution is critical to achieving motion-free breath-hold imaging of the liver, particularly during the dynamic phases of contrast enhancement. Dynamic contrast-enhanced liver imaging is typically performed with a 3D fat-suppressed T1-weighted gradient echo sequence. In most cases, the TR is kept as short as possible, with T1 contrast for this short TR maintained by use of a reduced flip angle. With the exception of newer dual-echo techniques, many manufacturers use an opposed-phase echo time for their dynamic liver sequence, leaving little room for further reduction of the TE at 1.5 T. Despite being reasonably optimized for breath-hold imaging, further improvements in temporal resolution can be gained over the “out-of-the-box” configuration, albeit at a price.
The simplest strategy to reduce scan time is to lower spatial resolution. Aside from potentially obscuring small structures and abnormalities, decreasing resolution can exacerbate truncation artifact (edge ringing that propagates through the images in the phase-encoding direction). The use of zero filling can help mitigate some of the effects of lower imaging matrices but can also exacerbate truncation artifact. Fortunately, truncation artifacts are rarely of clinical significance when imaging the liver using currently available pulse sequences and typical matrices (256 × 128 or higher). The SNR gained by lower spatial resolution can offset SNR lost by increasing the receiver bandwidth to further reduce acquisition time. Additional time savings can be achieved through the use of a reduced-phase field of view and partial Fourier methods.
Parallel Imaging
Parallel imaging has become a mainstay of hepatic MR imaging, allowing dramatic improvements in temporal resolution with a few trade-offs. These techniques save time by decreasing the number of individual phase-encoding steps necessary to create a complete image, which is accomplished by using the spatial information from individual coil elements in tandem with conventional gradient-based spatial encoding. These sequences are known as sensitivity encoding (SENSE), modified SENSE (mSENSE), or generalized autocalibrating partially parallel acquisition (GRAPPA), depending on the vendor and the method of data manipulation, either within k-space (GRAPPA) or within image space. In addition, these techniques vary with respect to calibration techniques. GRAPPA and mSENSE are autocalibrating, acquiring calibration data during image acquisition, whereas other techniques require dedicated calibration scans.
The use of parallel imaging can significantly reduce acquisition times, and is now considered routine for liver imaging. The reduction in scan time is related to the number of lines of k-space that are acquired in parallel during the phase encoding process (often referred to as the acceleration factor). This factor is higher for single-shot T2-weighted sequences (sometimes as high as 4). Signal, in this case, is maintained by the shorter echo train length. An acceleration factor of 2 or less is common for most abdominal applications, although higher factors are becoming more achievable on newer platforms. Some newer 3D sequences and coils allow for parallel imaging to be applied in the in-plane and through-plane direction simultaneously.
One downside of parallel imaging can be a reduction in SNR that worsens as the parallel imaging acceleration factor increases. This trend can be offset in many cases by adjustments in other parameters, although the signal gain through the use of gadolinium-based contrast media minimizes the impact of signal loss at typical acceleration factors of 2 or less. Another potential hazard associated with the use of parallel imaging is related to artifacts generated during image reconstruction. SENSE-based techniques reduce the number of phase encoding steps by acquiring data with a reduced phase field of view. The resulting aliased image is “unwrapped” using coil sensitivity profiles obtained during the calibration process. However, if the prescribed field of view is too small in the phase encoding direction, the unwrapping is incomplete and residual signal contaminates the central portion of the image. In addition, when there are too few phase-encoding steps acquired to maintain signal, or if the patient is large, noise amplification can be severe in the central portion of the image. When the field of view is increased in the phase direction in both cases, these artifacts can be reduced ( Fig. 2 ). Misregistration between the calibration scan and the image acquisition can also lead to artifacts ( Fig. 3 ), a common occurrence with breath-hold techniques when respiration is suspended inconsistently between the calibration scan and the image acquisition. Calibration artifacts can be corrected by recalibrating or by using one of the autocalibrating methods of parallel imaging. Table 1 provides an overview of the effects of changes in the some of the key parameters of sequence optimization.
| Parameter | Scan Time | Spatial Resolution | SNR | Artifact |
|---|---|---|---|---|
| Receiver bandwidth | An increase in bandwidth increases sampling rate and allows shorter TE, decreasing scan time | No direct effect on spatial resolution | SNR is decreased with increasing bandwidth | Water/fat misregistration is exacerbated at lower bandwidths |
| Acceleration factor (parallel imaging) | Increasing acceleration factor decreases scan time | No direct effect but may allow for improved spatial resolution for a given scan time | Increasing the parallel imaging factor decreases SNR | Artifacts related to aliasing and callibration are propagated in the phase-encoding direction |
| Field of view | Increasing field of view increases scan time if spatial resolution is kept constant | If the matrix is kept constant, voxel size will increase, resulting in decreased spatial resolution | If the matrix is kept constant, SNR increases due to larger voxel size | Most commonly associated with aliasing when the field of view is decreased and excludes anatomy. Artifact appears in the center of an image with parallel imaging if anatomy is excluded in phase-encoding direction |
| Matrix | Scan time is increased with a finer matrix in the phase-encoding axis | Spatial resolution is increased with an increase in matrix for constant field of view | SNR is decreased with smaller voxel size | Truncation artifact may be seen with marked reduction in matrix |
| Slice thickness | Increasing slice thickness decreases scan time for constant anatomic coverage | Through-plane resolution is decreased when slice thickness is increased | SNR increases with increasing slice thickness | Degradation of multiplanar reconstructions and worsened volume averaging effects with increased slice thickness |
| Number of signal averages (NEX, NSA) | Increasing NSA increases scan time | No effect on spatial resolution | SNR increases with increasing NSA | May introduce image blurring with higher NSA |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree