Fig. 7.1
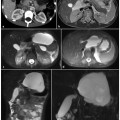
Diffuse (a–c) and focal (d–f) chronic pancreatitis. Axial T1-weighted fat-suppressed image before (a, d) and after (b, e) intravenous contrast medium administration during the pancreatic phase. c, f Magnetic resonance cholangiopancreatography (MRCP). a–c The diffuse chronic pancreatitis is characterized by a decreased anterior-posterior diameter of the pancreatic gland, which shows a low signal intensity compared with the liver on a fat-suppressed T1-weighted image (a) and a reduced enhancement during the pancreatic phase (b). c MRCP shows a markedly irregular and dilated main pancreatic duct, with evidence of multiple side branches and several cysts. d–f Focal chronic pancreatitis of the body-tail in a patient who had had a previous episode of acute pancreatitis, with consequent focal rupture of the duct of Wirsung (f, short arrow). Typical findings of chronic pancreatitis have developed upstream the rupture point. The main pancreatic duct changes its caliber sharply at this level. In addition there is a clear difference between the pancreatic head (d, e, arrow), which shows regular dimensions, signal intensity and enhancement, and the body-tail (d, e, arrowhead), which presents reduced anterior-posterior diameter, low signal intensity on the fat-suppressed T1-weighted image and reduced enhancement during the pancreatic phase. f MRCP demonstrates a mildly dilated and irregular duct of Wirsung throughout the body-tail, with multiple dilated secondary branches. A normal duct of Wirsung caliber is preserved within the pancreatic head
Signal of the gland: the pancreatic signal decreases on T1-weighted fat-suppressed images; this is more evident with increasing loss of exocrine capability of the gland (Fig. 7.1a, d).
Perfusion of pancreatic gland: this should be evaluated on serial contrast-enhanced images. In chronic pancreatitis, the typical arterial capillary peak enhancement is replaced by delayed enhancement because of the presence of fibrosis (Fig. 7.1b, e). The gland reaches its maximum enhancement in the venous phases in a gradual fashion.
Standard MRCP images allow evaluation of ductal changes and observation of the presence of an obstructive cause of chronic pancreatitis.
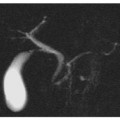
MRCP findings in chronic pancreatitis can include: biliary and pancreatic ductal dilatation, strictures, and irregularities in the main pancreatic duct and/or ectasia of its side branches. These features have been evaluated and classified according to Cambridge criteria for chronic pancreatitis (Fig. 7.2) [12]:
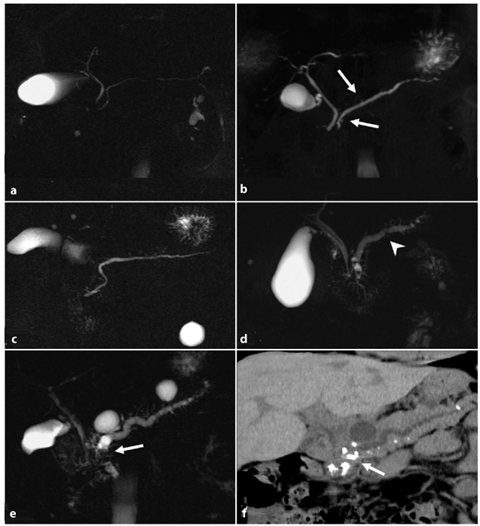
Cambridge 1 (normal pancreas): no abnormalities of pancreatic ductal anatomy are detected (Fig. 7.2a).
Cambridge 2 (equivocal findings): there is dilatation/obstruction of less than three side branches, with a normal main pancreatic duct (Fig. 7.2b).
Cambridge 3 (mild disease): more than three side branches are dilated, with a normal main pancreatic duct (Fig. 7.2c).
Cambridge 4 (moderate disease): Cambridge category 3 features plus stenosis and dilatation of the main pancreatic duct (Fig. 7. 2d).
Cambridge 5 (severe disease): Cambridge category 3 and 4 features plus additional calculi, obstructions, cysts and/or stenosis of the main pancreatic duct (Fig. 7.2e).

Fig. 7.2
Cambridge classification of chronic pancreatitis when applied to magnetic resonance cholangiopancreatography (MRCP) images. a Cambridge 1 (normal pancreas): unremarkable main pancreatic duct (MPD). b Cambridge 2 (equivocal findings): less than three secondary ducts dilated (arrows), associated with a normal MPD. c Cambridge 3 (mild chronic pancreatitis): more than three secondary ducts dilated, associated with a normal MPD. d Cambridge 4 (moderate chronic pancreatitis): category 3 criteria plus multiple focal stenosis (arrowhead) and irregular dilatation of the MPD. e Cambridge 5 (severe chronic pancreatitis): all the previous category findings plus additional calculi-filling defects on MRCP images (arrow): obstructions, cysts and/or stenosis of the MPD. f Cambridge 5 (severe chronic pancreatitis): multidetector computed tomography curvilinear multiplanar reconstruction confirms several calcifications, mainly within the pancreatic head (arrow), corresponding to the multiple filling defects detected on the MRCP images (e). The upstream dilated MPD is also visible
However, none of the above-described parenchyma parameters are useful for detecting abnormal pancreatic exocrine function.
7.1.2 Role of Secretin-Stimulated MRCP in Assessing Chronic Pancreatitis
Secretin-stimulated MRCP (s-MRCP) is considered to be a “tubeless direct test” for the measurement of pancreatic secretion volume [13] and the assessment of exocrine function of the gland. Furthermore, s-MRCP provides more detailed information about ductal changes.
Secretin is a 27-amino-acid polypeptide hormone produced by duodenal mucosa and secreted in response to the increased acid level in the duodenal lumen after a meal. It induces pancreatic bicarbonate-rich fluid secretion into the duodenum and increases the tone of the sphincter of Oddi [14]. These effects result in temporary distension of the pancreatic ducts.
s-MRCP requires the intravenous injection of synthetic human secretin, which should be administered over 1 min period in order to avoid potential abdominal pain. The injection dosage is 0.2μg/kg body weight in adults. A baseline scan must be obtained at the beginning of the injection, followed by a coronal half-Fourier rapid acquisition with relaxation enhancement (RARE) image every 30 s for 10 min [11, 14]. In healthy subjects, the maximal distension of the pancreatic duct occurs 4–10 min after secretin injection, with a peak 2–3 min after the injection [15, 16]. The caliber of the main pancreatic duct after secretin injection is expected to be at least 1 mm greater than the baseline caliber.
Although a system using s-MRCP to grade different stages of chronic pancreatitis has not yet been defined, several findings associated with the disease must be taken in account:
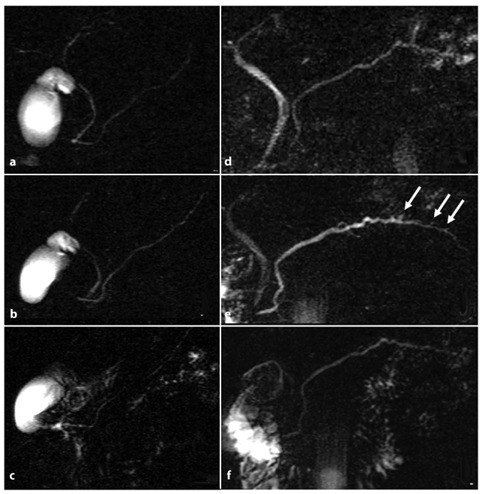
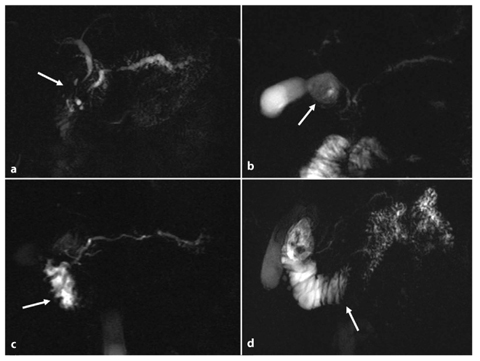
Inadequate pancreatic duct distension. Impaired ductal compliance is diagnosed when the dilatation of stimulated main pancreatic duct is less than 1 mm (Fig. 7.3a–c). It is important to exclude a previous sphincterectomy, as there is no ductal response to secretin if the sphincter is incompetent [17].
An increased number or new visualization of side-branch ectasias. This is a very important feature, as side-branch ectasias have been proved to correlate with the early stages of chronic pancreatitis, and they are also seen in cases of normal pancreatic exocrine function [18–20]. An increased number or new detection of sidebranch ectasias after secretin stimulation compared with baseline MRCP (Fig. 7.3d–f) facilitates early diagnosis of mild fibrosis in the gland [11].
Diminished duodenal filling after secretin stimulation. The pancreatic exocrine reserve can be indirectly estimated by the amount of pancreatic fluid that is excreted into the duodenum and this can be visualized during s-MRCP [18, 21, 22]. Duodenal filling is graded according to duodenal anatomy:
Grade 0: no fluid in the duodenum after secretin stimulation (Fig. 7.4a).
Grade 1: filling remains limited to the duodenal bulb (Fig. 7.4b).
Grade 2: fluid is visualized in the first and second portions of the duodenum up to the genu inferius (Fig. 7.4c).
Grade 3: fluid reaches the third portion of the duodenum (Fig. 7.4d).

Fig. 7.3
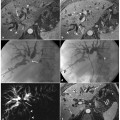
Role of secretin-stimulated magnetic resonance cholangiopancreatography in the diagnosis of chronic pancreatitis. a–c Magnetic resonance cholangiopancreatography (MRCP) images obtained before (a), and 4 min (b) and 10 min (c) after, intravenous administration of secretin. A 37-year-old woman with recurrent episodes of mild acute pancreatitis presents a pancreas divisum ductal configuration, better visualized after the administration of secretin (b). There is no relevant distension of the main pancreatic duct in response to the secretin stimulation, and there is associated reduced duodenal filling (grade 1) 10 min (c) after the injection. These findings are in line with pancreatic exocrine insufficiency. e–f MRCP findings in chronic pancreatitis before (d), and 5 min (e) and 10 min (f) after, the intravenous administration of secretin. The Santorini duct is visualized in response to secretin. There is an increase in signal intensity and caliber of the duct of Wirsung, which also appears irregular within the body-tail. At this level, detection of side branch ectasias (arrows) is made possible by secretin. At 10 min after secretin injection (f), the duct of Wirsung shows reduced caliber, returning to its initial size, and there is a regular duodenal filling (grade 3)

Fig. 7.4
Pancreatic exocrine function assessment on secretin-stimulated magnetic resonance cholangiopancreatography (s-MRCP) images: duodenal filling grading system. a–d MRCP images acquired 10 min after secretin injection. a Grade 0: severe chronic pancreatitis associated with exocrine insufficiency; there is no fluid within the duodenum (arrow). b Grade 1: moderate chronic pancreatitis and exocrine insufficiency; there is no pancreatic fluid overflowing the duodenal bulb (arrow). c Grade 2: mild chronic pancreatitis; pancreatic fluid can be seen in the first and second portions of the duodenum up to the genu inferius (arrow). d Grade 3: preserved exocrine pancreatic function; pancreatic fluid has reached the third portion of the duodenum (arrow)
A diminished pancreatic exocrine function is found in cases of grade <3; this technique has been validated in a comparative study using an intraductal secretin test [18].
It is clear that MRI in conjunction with s-MRCP represents a “one stop shop examination” [23] for global assessment of chronic pancreatitis in different stages of the disease: recurrent, early chronic pancreatitis and advanced chronic pancreatitis.
7.2 Main Causes of Primary Chronic Pancreatitis
As previously mentioned, the form of chronic pancreatitis that is not caused by ductal obstruction is the so-called primary or nonobstructive chronic pancreatitis. Autoimmune chronic pancreatitis and chronic pancreatitis associated with gene mutation are the main causes of this form of the disease.
7.2.1 Chronic Pancreatitis Associated With Genetic Mutations
Hereditary chronic pancreatitis is a rare form of early-onset chronic pancreatitis, and is associated with several gene mutations. The majority of cases are related to mutation of the cystic fibrosis gene (CFTR), the serine protease inhibitor Kazal type 1 gene (SPINK1), the cationic trypsinogen gene (PRSS1) and the gene encoding keratin 8.
Genetic forms of the disease do not differ from other forms of chronic pancreatitis, with the exception of a younger age at diagnosis and a slower progression. Patients do not usually have other risk factors for pancreatitis. The mean age of onset is 19 years [24] and the progression to chronic pancreatitis takes about 7 years, with increasingly numerous episodes of acute pancreatitis. Early diagnosis is fundamental so that medical or endoscopical treatment can be commenced as soon as possible, in order to delay the progression of the disease. Genetic tests are essential for diagnosis, but imaging is also important for both diagnosis and follow-up because of the high risk of pancreatic cancer (2.8%) in these patients [25, 26]. Standard MRI and MRCP can assess several morphological changes in both the pancreatic gland and the ductal system.
Typical MRI and MRCP findings in early disease are: normal or increased dimension of the pancreatic gland in the early phase; fibrosis of the pancreatic parenchyma, which appears hypointense on fat-suppressed T1-weighted gradient-echo images, when compared with the liver; delayed perfusion of the gland, which therefore appears hypointense on T1-weighted fat-suppressed gradient-echo post-contrast images in the pancreatic phase, compared with the normal parenchyma; normal or quite dilated main pancreatic duct, without calculi.
Typical MRI and MRCP findings in advanced disease are: a decrease in pancreas dimension (about 2 years after diagnosis), which is less than that seen in chronic obstructive pancreatitis, with a late onset of atrophy; the main pancreatic duct is irregularly dilated, with multiple calculi (MRI does not allow direct visualization of the calcifications, which look like endoluminal defects).
Pre-contrastographic computed tomography (CT) examination is strongly required for characterization of calculi in the hereditary form of chronic pancreatitis. In fact, it has been proved that the finding of a “Bull’s eye” with a hypodense central core is highly specific for this disease [7].
7.2.2 Autoimmune Chronic Pancreatitis
Autoimmune pancreatitis is a chronic inflammation of the pancreas associated with an autoimmune process. It typically involves patients over 55 years of age, with a slight prevalence for females, and often presents with symptoms that overlap with those of other forms of chronic pancreatitis. A coexisting focal or systemic autoimmune disease (Crohn’s disease, ulcerative colitis, Sjögren’s syndrome, systemic lupus erythematosis, Reiter syndrome, etc.) is present in about 50% of patients. There is no relationship between the disease and smoking or alcohol abuse [7, 16].
The presence of a rich inflammatory cellular infiltration (lymphocytes, plasma cells and granulocytes), typically localized around the affected pancreatic duct, is specific for the disease. The infiltration can be focal or diffuse, and can reflect different stages of the disorder [27]. Acinar atrophy and marked fibrosis are reached in more advanced phases. The portal vein and the biliary system can be involved by obliterative phlebitis and wall inflammation, respectively [28].
Clinically, the disease can present as an episode of acute pancreatitis (diffuse form of autoimmune chronic pancreatitis) or, more frequently, as sudden painless jaundice (focal form). In the latter case, differential diagnosis with adenocarcinoma can be very difficult. As autoimmune pancreatitis responds to steroid therapy with a clinical remission within a few weeks, this therapy is used as a diagnostic criterion for autoimmune chronic pancreatitis. For this reason, the steroid response must be investigated in all suspected cases of autoimmune pancreatitis in order to potentially avoid invasive surgery.
Laboratory data associated with autoimmune chronic pancreatitis are elevated levels of serum γ-globulin and IgG/IgG4, or the presence of autoantibodies.
Typical MRI findings of autoimmune chronic pancreatitis on standard MRI are [29, 30]: focal or diffuse pancreatic enlargement with sharp borders (Fig. 7.5a, d); focal or diffuse abnormal signal intensity, homogenous hypointensity on gradient-echo T1-weighted images and hyperintensity on turbo-spin-echo T2-weighted images; decreased arterial enhancement with a homogenous delayed contrast uptake (Fig. 7.5b–c, e–f); hypointense capsule-like rim on T2-weighted images (Fig. 7.5a, d) (not present in all cases) and the rim corresponds to peri-pancreatic stranding visible in CT images [31].
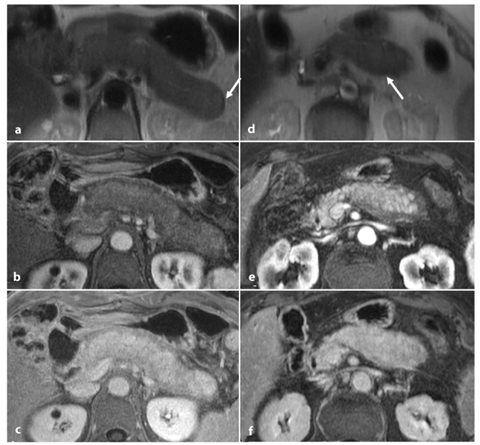

Fig. 7.5
Diffuse (a–c) and focal (d–f) autoimmune chronic pancreatitis. Axial half-Fourier acquisition single-shot turbo- spin-echo (HASTE) T2-weighted images (a, d), and axial T1-weighted fat-suppressed images after intravenous contrast medium administration during the pancreatic phase (b, e) and the delayed phase (c, f). a–c Typical appearance of diffuse autoimmune pancreatitis: the overall pancreatic gland is enlarged with sharp borders and a hypointense capsulelike rim (a, d, arrow) on the T2-weighted images. There is decreased enhancement on the arterial pancreatic phase (b), with increased homogeneous contrast uptake during the delayed phase (c). d–f The same findings are demonstrated within the pancreatic body itself for the focal form of autoimmune pancreatitis
In MRCP images, the main pancreatic duct is typically not dilated, as it is compressed by the cramped cellular material all around it. Furthermore MRCP can show diffuse and irregular narrowing of the main pancreatic duct. It is also important to evaluate the involvement of the biliary ducts, which are frequently associated (autoimmune cholangitis, Fig. 7.6a, b).
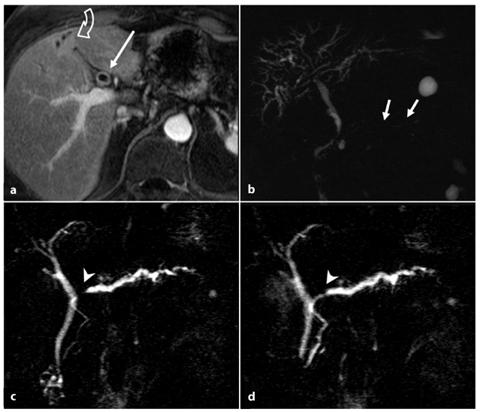

Fig. 7.6
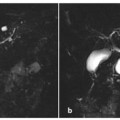
Autoimmune pancreatitis: associated autoimmune cholangitis (a, b) and the “duct-penetrating sign” (c, d). a, b Axial T1-weighted fat-suppressed images after intravenous contrast medium administration during the pancreatic phase (a), and magnetic resonance cholangiopancreatography (MRCP) images (b). These images are from the same patient as in Fig. 7.5 d–f and they show cholangitis associated with focal autoimmune pancreatitis. The patient had had a previous cholecystectomy. In the current situation, mild dilatation of the intrahepatic and extrahepatic bile ducts has developed during the episode of autoimmune pancreatitis (b), with thickening of the bile duct walls, which enhance after contrast medium administration (a, arrow). The duct of Wirsung has a thread-like caliber, with multiple focal stenosis (b, arrows). Some areas of hepatic arterialization are seen during the pancreatic phase (a, curved open arrow), adjacent to the dilated ducts. c, d MRCP images obtained from a patient with focal autoimmune pancreatitis before (c) and 1 minute after (d) intravenous administration of secretin. The duct of Wirsung shows a short stricture at the passage between the head and the body (c,d, arrowhead), at the level of the autoimmune inflammatory process. d The stenosis resolves after secretin stimulation, a finding that is in line with a benign etiology of the stricture (duct-penetrating sign, arrowhead)
s-MRCP evaluation can be useful to better visualize the pancreatic duct, which dilates after stimulation. This is particularly important in cases of focal autoimmune chronic pancreatitis with short ductal stricture, which can mimic malignancy. If the stricture resolves after secretin stimulation — the socalled “duct-penetrating sign” (Fig. 7.6c, d) — it is possible to rule out malignancy [32].
7.3 Main Causes of Secondary Chronic Pancreatitis
The form of chronic pancreatitis determined by an obstruction of the main pancreatic duct is known as secondary or obstructive chronic pancreatitis.
There are many possible causes of obstruction of the pancreatic ductal system, of which the main ones are: (1) sphincter of Oddi dysfunction, which can be primary or secondary to acute or chronic biliary stone disease; (2) neoplastic causes; (3) malformations; (4) paraduodenal pancreatitis (cystic dystrophy of the duodenal wall/groove pancreatitis); (5) necrotizing acute pancreatitis.
7.3.1 Pancreatitis Associated with Sphincter of Oddi Dysfunction or Biliary Lithiasis
Stenosis of the sphincter of Oddi represents the most frequent cause of chronic pancreati Stenosis of the sphincter of Oddi represents the most frequent cause of chronic pancreatitis (40% of cases) [35]. This is caused by calculi and biliary sludge moving through the papilla several times, resulting in severe damage related to inflammation and fibrosis. The main consequence is the obstruction of the main pancreatic duct, which dilates gradually and uniformly. Dilatation of the side branches and formation of intraductal calculi develop later on during the disease (36%) [36].
Standard MRI shows non-specific alterations that are typical of all forms of chronic pancreatitis, but it does facilitate diagnosis of sphincter dysfunction.
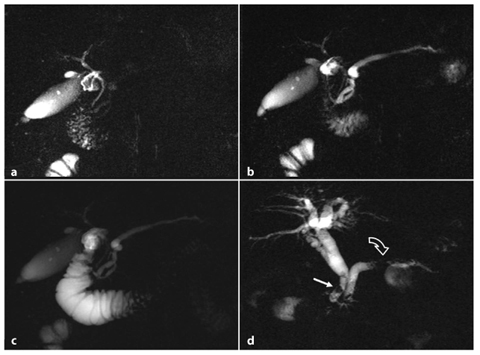
s-MRCP is crucial for diagnosis: normal dilatation of pancreatic ducts occurs, but pancreatic secretions cannot reach the duodenal lumen, so the caliber of the main pancreatic duct persists in a dilated state for 10 min after secretin administration (Fig. 7.7a–c).
Get Clinical Tree app for offline access

Fig. 7.7
Failed caliber recovery of the main pancreatic duct 10 min after secretin injection (a–c), and main biliary duct lithiasis (d). a–c Secretin-stimulated magnetic resonance cholangiopancreatography images acquired at baseline (a), and 3 min (b) and 10 min (c) after secretin injection. a The main pancreatic duct (MPD) at baseline in a patient with mild chronic pancreatitis. b The MPD appears homogeneously dilated in response to secretin stimulation. c The MDP has not recovered to the baseline condition and remains dilated. d Chronic pancreatitis associated with biliary lithiasis. There are several filling defects (arrow




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree