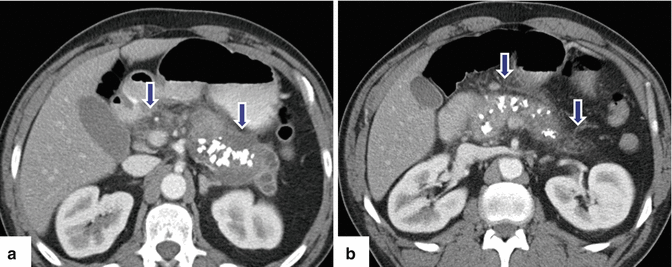
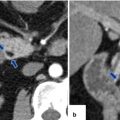
Fig. 15.1
Chronic pancreatitis, macroscopic appearance. Intraoperative photograph (a) shows a small, fibrotic pancreas (arrows), and specimen photograph (b) shows an enlarged, firm pancreas (arrows)
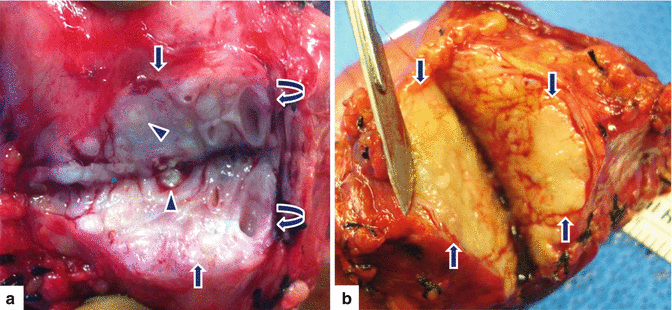
Fig. 15.2
Chronic pancreatitis, macroscopic appearance. Photographs of two bivalved Whipple specimens demonstrate (a) a solid, white, hard mass-like area with fibrosis (arrows), ectatic ducts (curved arrows), and calcifications (arrowheads), and (b) a yellow. firm, ill-defined mass-like area
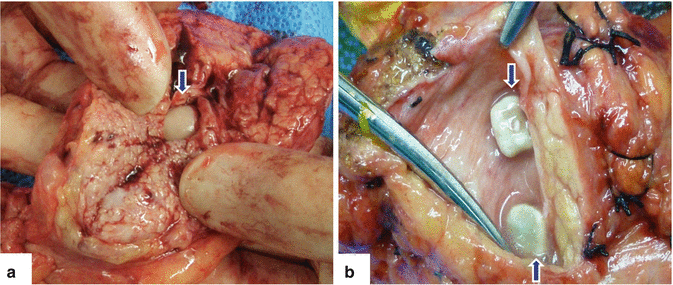
Fig. 15.3
Chronic pancreatitis, pancreatic calcifications (calculi). Photographs of two bivalved surgical specimens (a, b) reveal intraductal calcifications (arrows)
Hard, shrunken, fibrotic pancreas
Dilated pancreatic ducts
Visible calcified concretions and occasional pseudocysts
15.4.2 Microscopic Appearance
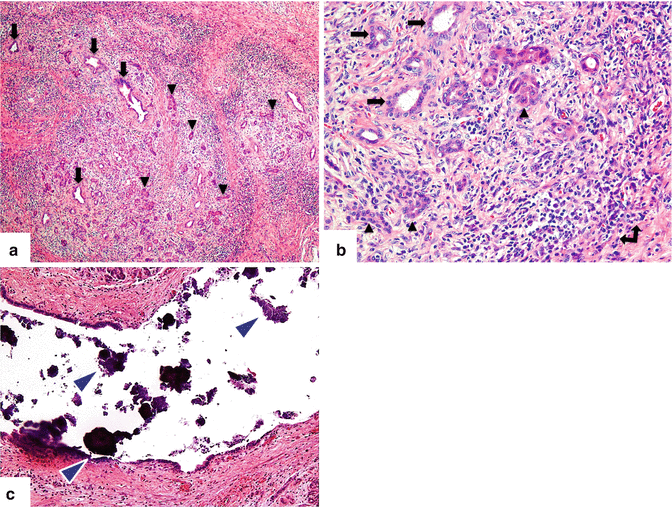
Fig. 15.4
Chronic pancreatitis, microscopic appearance. Photomicrographs (a) (H&E, 4×) show retention of the lobular architecture accompanied by fibrosis and chronic inflammation, pancreatic ducts (arrows) and atrophic acini (arrowheads), and (b) (H&E, 40×) acinar atrophy (arrowheads), atypical ducts (arrows), and lymphoplasmacytic inflammatory infiltrate (double-headed arrow) with collagenous fibrosis. Microphotograph (H&E, 20×) (c) shows marked dilation of a pancreatic duct with intraluminal calcifications (arrowheads)
Collagenous fibrosis with acinar atrophy and retention of the usual lobular architecture.
Islet cells can be spared in mild cases or lost in severe cases.
Mononuclear cell inflammatory infiltrate.
Variable obstruction of pancreatic ducts of all sizes.
Calcifications are common.
Early lesions are distributed in patches.
Each inflammatory attack can cause foci of fat necrosis that might lead to both pseudocysts and fibrosis.
Fibrosis may diffusely affect the gland but occasionally is distributed unevenly, leaving the lobular pattern of the organ preserved in some areas.
The severity of the ductal changes depends on the extent of surrounding fibrosis.
Perilobular fibrosis causes ductal distortion and dilatation with formation of retention cysts.
Main duct may only be focally obstructed and/or dilated, or diffusely involved with irregular dilatation and distortion throughout.
Intraductal calculi (80 %).
Calculi consist of calcium carbonate.
Size: ≤1 mm to ≥1 cm in diameter.
They may be impacted in the ducts
In some cases, they may disappear during the course of the disease.
Practical Pearls
Fibrosis of the pancreatic head may be associated with stenosis of the common bile duct.
Duodenal stenosis may be seen secondary to the presence of fibrosis or pseudocysts in the pancreatic head.
15.5 Etiology
Alcohol abuse 70 % (most common cause)
Chronic alcoholic pancreatitis
Presents in the fourth or fifth decade of life
Mainly affects men
Pancreatic duct obstruction
Pancreatic divisum
Post-traumatic scarring
Post-duct destruction in severe attack
Stones
Pseudocysts
Tumors (adenocarcinoma, IPMN)
Idiopathic pancreatitis
Two peaks: early onset (second decade) and late onset (sixth decade)
Accounts for 10–25 % of all cases of chronic pancreatitis
Equal gender distribution
Groove pancreatitis
Autoimmune pancreatitis
Tropical pancreatitis
Presents between 20 and 30 years
Central Africa, Brazil, and India
Associated with malnutrition in childhood
Occurs in adolescents
Formation of pancreatic calculi
Mutation in SPINK1 gene
Cystic fibrosis
Hereditary
Manifests at around 10 years
Genetic
Systemic disease such as lupus erythematous
Hypertriglyceridemia
Hyperparathyroidism
Cigarette smoking
15.6 Clinical Presentation
Patients with chronic pancreatitis usually fall into one of the following four groups:
Acute or recurrent attacks of pancreatitis.
Constant abdominal pain.
Symptoms and signs of local complications of the disease (pseudocyst, obstruction of adjacent organs, or vascular thrombosis).
Complaints that suggest exocrine or endocrine pancreatic failure or both.
Interval from first attack of alcoholic pancreatitis to steatorrhea is around 13 years.
Diabetes may precede, begin concurrently, or start after steatorrhea.
Practical Pearls
CP may be asymptomatic over long periods of time.
Only 10 % of heavy drinkers develop the disease.
The disease develops around 10 years after alcohol abuse.
Can present with a fibrotic mass or with symptoms of pancreatic insufficiency without pain.
To develop steatorrhea, >95 % of pancreatic acini have to be lost.
PAIN is the overriding symptom in the majority of the patients.
Pain is wearying and occurs in episodes that may last up to 1 week or be persistent.
Pain can be so severe that patients fear of food becomes chronic, thereby causing weight loss.
Begins in the epigastrium and moves through to the dorsal spine or localizes to the left hypochondrium, radiating to the left infrascapular region.
Pain is sometimes associated with nausea and vomiting.
Pain can be partially eased by sitting up and leaning forward or by the application of local heat in the epigastrium or the dorsal spine.
Practical Pearls
Some patients with chronic pancreatitis do not present with abdominal pain. These cases usually occur in elderly patients with idiopathic disease or patients with autoimmune pancreatitis.
Pain in CP diminishes markedly once the disease “burns out.”
Patients often become addicted to narcotic analgesics.
Findings Suggestive of Exocrine Insufficiency
Diarrhea, steatorrhea, weight loss, metabolic bone disease, and vitamin or mineral deficiency
Findings Suggestive of Endocrine Insufficiency
Fasting plasma glucose level ≥126 mg/dl
2-h oral glucose tolerance test result >200 mg/ dl
Hemoglobin A1c ≥6.5 %
Additional Clinical Findings
Erythema ab igne is a useful indicator for the diagnosis of chronic pancreatitis.
Jaundice suggests autoimmune pancreatitis or a superimposed adenocarcinoma.
Constipation and meteorism suggest dependence on narcotic analgesics.
Complications
Pseudocysts
Venous thrombosis (splenic, portal, and superior mesenteric veins)
Pseudoaneurysm (splenic, gastroduodenal, and hepatic arteries)
Biliary obstruction
Duodenal or gastric obstruction
Pancreatic ascites
Pleural effusion
15.7 Laboratory Evaluation
Routine laboratory tests might reveal incipient diabetes, hyperlipidemia, or hypercalcemia.
Abnormal liver function profile may suggest
Alcoholic liver disease
Nonalcoholic steatohepatitis
Sclerosing cholangitis
Metastases (pancreatic carcinoma)
Biliary stones
Constriction of the common bile duct
Serum amylase and lipase levels tend to be normal in patients with CP.
15.8 Imaging
Preferred imaging modalities:
Contrast-enhanced computed tomography (CECT)
Magnetic resonance (MR) and MR cholangiopancreatography (MRCP)
Endoscopic ultrasonography (EUS)
15.8.1 Plain Abdominal Films (Fig. 15.5)
Findings
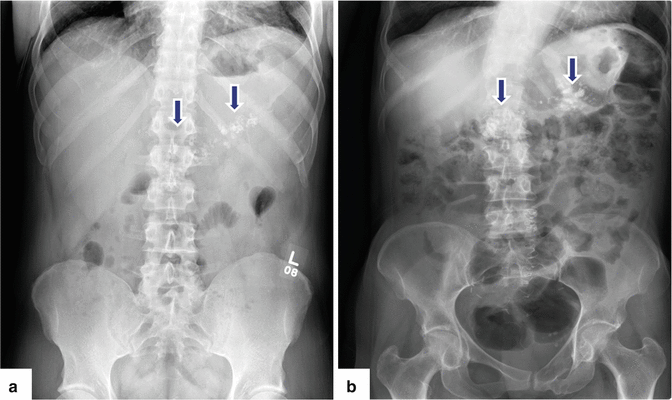

Fig. 15.5
Chronic pancreatitis on plain abdominal radiographs. Abdominal radiographs (a, b) of two alcoholic patients show multiple coarse calcifications in the topography of the pancreas (arrows)
Calcifications in the distribution of the pancreatic gland (25–59 %)
Coarse or punctate (represent intraductal calculi, either in the main pancreatic duct or in the smaller pancreatic duct radicles)
Diffuse or focal distribution
Osteomalacia secondary to malabsorption
Practical Pearls
The presence of pancreatic calcifications is considered pathognomonic for alcoholic pancreatitis.
15.8.2 Ultrasound (US) (Figs. 15.6–15.12)
Findings
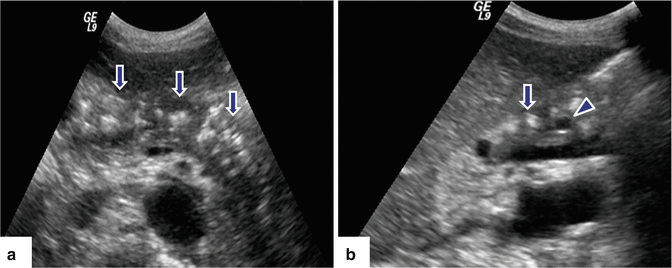
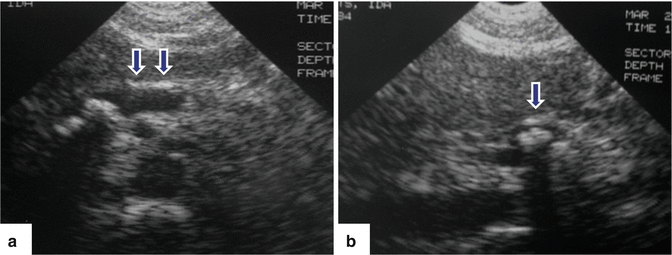
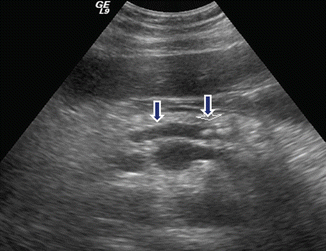
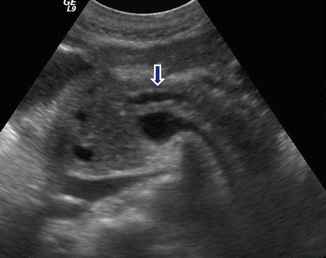
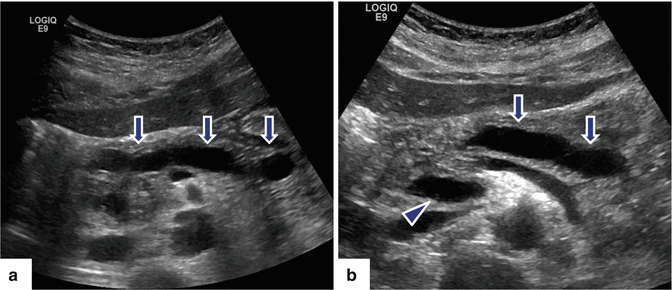
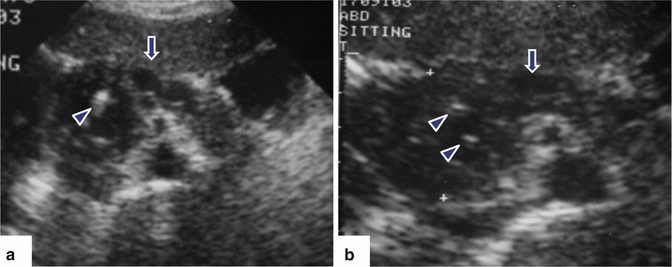
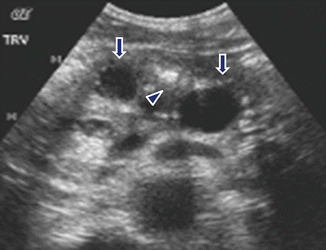

Fig. 15.6
Chronic pancreatitis on US. A 45-year-old male with epigastric pain and history of alcohol abuse. Transverse (a) and sagittal (b) images of the pancreas show multiple hyperechoic non-shadowing foci in the pancreatic parenchyma (arrows) associated with mild dilatation of the pancreatic duct. Finding is better appreciated in sagittal view (arrowhead)

Fig. 15.7
Chronic pancreatitis on US. A 52-year-old female with history of chronic upper abdominal and back pain and long history of alcohol abuse. Transverse (a) and sagittal (b) images of the pancreas demonstrate a marked dilated main pancreatic duct in the body of the pancreas (arrows) with calcifications and parenchyma atrophy

Fig. 15.8
Chronic pancreatitis on US. A 62-year-old male with history of mild upper abdominal pain. Transverse image of the pancreas demonstrate a mild, dilated main pancreatic duct containing calculi (arrows)

Fig. 15.9
Chronic pancreatitis on US. A 52-year-old female with a long history of alcohol abuse and intermittent abdominal and back pain. Transverse image of the pancreas show a beaded appearance of the dilated main pancreatic duct in the body of the pancreas (arrow)

Fig. 15.10
Chronic pancreatitis on US. A 19-year-old male patient with repetitive attacks of pancreatitis. Transverse images of the pancreas (a, b) reveal a marked, smooth, dilated, tortuous pancreatic duct in the body and tail of the pancreas with abrupt cut off in the neck (arrows). Note the presence of a small pseudocyst around the head of the pancreas (arrowhead). MRCP performed in this patient showed a pancreatic divisum with stenosis of the proximal dorsal duct

Fig. 15.11
Chronic pancreatitis on US. A 40-year-old male with chronic back pain. Transverse images of the pancreas (a, b) demonstrate a prominent pancreatic head with heterogeneous echogenicity. Note the presence of small echogenic foci in this region (arrowheads) and dilatation of the pancreatic duct in the body of the pancreas (arrows)

Fig. 15.12
Chronic pancreatitis on US. A 49-year-old male with history of alcohol abuse, repetitive attacks of acute pancreatitis, and upper back pain. Transverse image shows two cystic lesions (pseudocyst) (arrows) and multiple echogenic foci in the body of the pancreas (arrowhead)
Transabdominal US
Dilatation of the pancreatic duct
Diffuse or focal echogenic foci without shadowing (pancreatic ductal stones)
Parenchymal atrophy
Pancreatic parenchyma: hyperechoic or heterogeneous
Focal, heterogeneous mass (fibrosis), most common in the pancreatic head
Pseudocysts
Splenic vein thrombosis
Common bile duct obstruction
Endoscopic US (EUS)
Parenchymal oval hypoechoic areas smaller than 1 mm and separated by fibrous septa (EUS)
Practical Pearl
Ultrasound may demonstrate a normal pancreas in the presence of established chronic pancreatitis.
15.8.3 Computed Tomography (CT) (Figs. 15.13–15.33)
Findings
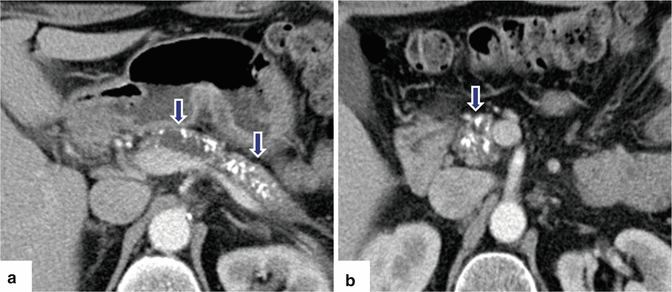
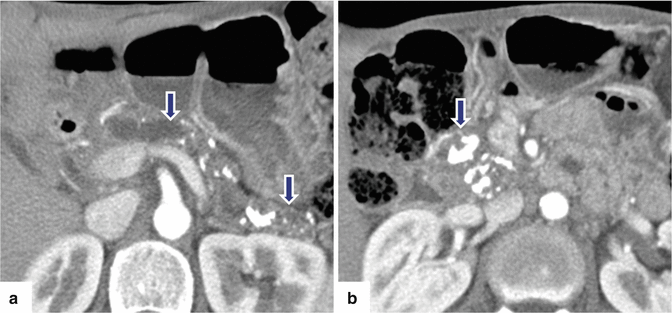
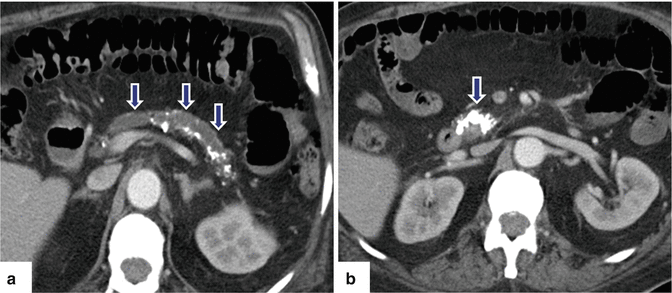
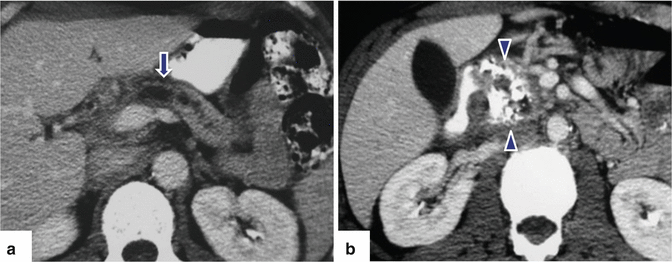
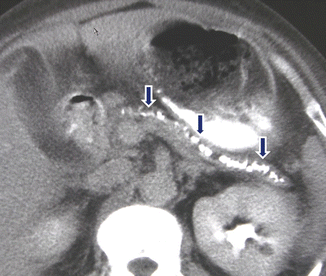
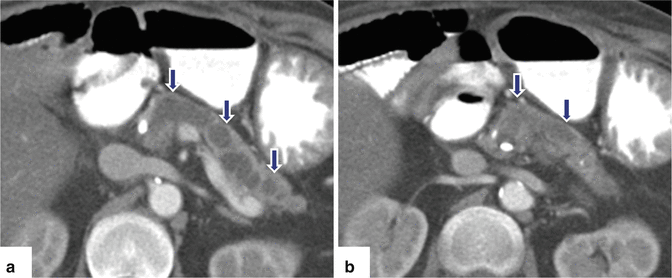
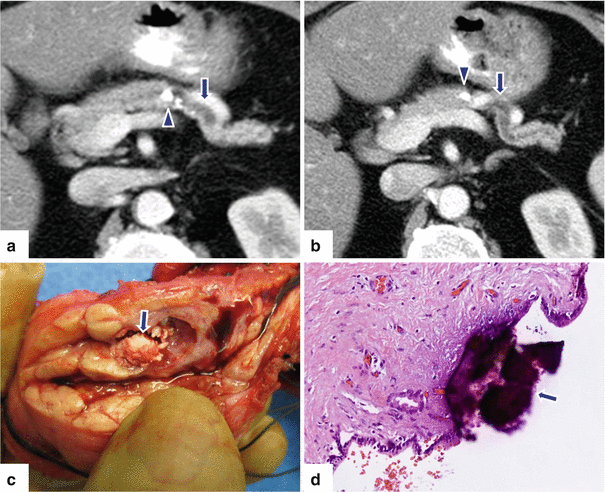
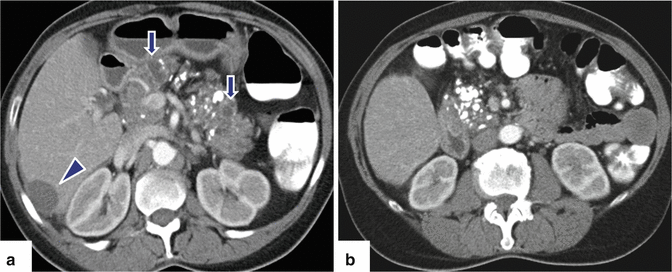
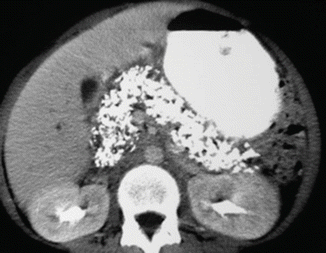
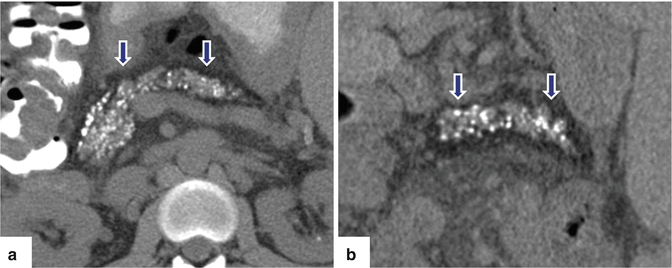

Fig. 15.13
Chronic pancreatitis on CT. A 57-year-old male with alcohol abuse. CECT axial images (a, b) show a dilated pancreatic duct, multiple calcifications (arrows) throughout the pancreas, and parenchymal atrophy

Fig. 15.14
Chronic pancreatitis on CT. A 48-year-old male with medical history of type I diabetes, alcoholism, and multiple episodes of pancreatitis. CECT axial images (a, b) demonstrate marked atrophy of the pancreas, irregular dilatation of the main pancreatic duct (arrowhead), and multiple calculi, more prominent in the head (arrows)

Fig. 15.15
Chronic pancreatitis on CT. A 73-year-old with history of alcohol abuse. CECT axial images (a, b) reveal a prominent dilated main pancreatic duct (arrows), marked parenchymal atrophy, and multiple calcifications in the pancreas

Fig. 15.16
Chronic pancreatitis on CT. A 45-year-old female with idiopathic pancreatitis. CECT axial (a, b) images show a dilated, irregular duct in the body and tail of the pancreas (arrow), multiple calcifications in the head (arrowheads), and atrophy of the parenchyma

Fig. 15.17
Chronic pancreatitis on CT. A 53-year-old female patient with repetitive attacks of pancreatitis. CECT axial image shows marked atrophy of the pancreas and multiple intraluminal calculi in the main pancreatic duct (arrows)

Fig. 15.18
Chronic pancreatitis on CT. A 52-year-old female with history of lymphoma and alcohol abuse. CECT axial images (a, b) demonstrate an irregular, markedly dilated main pancreatic duct (arrows), atrophy of the pancreas, and a few coarse calcifications in the head and tail of the pancreas

Fig. 15.19
Segmental chronic pancreatitis on CT. A 71-year-old female with recurrent episodes of left upper quadrant pain. CECT axial (a, b) images reveal obstruction of the distal pancreatic duct by intraluminal calcifications (arrowheads). Note the atrophy of the pancreas distal to the obstruction. A distal pancreatectomy was performed. Photograph of the bivalved specimen (c) corroborates the presence of calculi in the pancreatic duct (arrow). Histologic section (d) (H&E, 10×) reveals coarse intraductal calcification (arrow) with underlying marked fibrosis

Fig. 15.20
Chronic pancreatitis on CT. A 52-year-old female with recent diagnosis of ovarian carcinoma and medical history of alcohol abuse. Incidental finding on abdominal CT performed for staging. CECT axial images (a, b) demonstrate multiple coarse calcifications throughout the pancreas associated with small pseudocysts (arrows). Note a subcapsular cystic mass in the right lobe of the liver corresponding to a metastatic deposit from the ovarian malignancy (a) (arrowhead)

Fig. 15.21
Chronic pancreatitis on CT. A 42-year-old male with history of alcohol, drug abuse and chronic abdominal pain. CECT axial image demonstrates innumerable calcifications throughout the pancreas

Fig. 15.22
Chronic pancreatitis on CT. A 37-year-old male with history of cystic fibrosis. Non-contrast CT axial images (a, b) reveal multiple, small pancreatic calcifications and parenchyma atrophy with diffuse fatty infiltration (arrows) of the pancratic parenchyma