Fig. 36.1
Structures of glutaryl phenylalanine hydroxylmethyl rhodamine green (gPhe-HMRG). The gPhe-HMRG probe is activated with chymotrypsin secreted in pancreatic juice and emits HMRG fluorescence
Figure 36.2 shows the trends of the median fluorescence intensity (FI) obtained following addition of the chymotrypsin probe to drained fluid samples in previous series of pancreatic resection. The FI of pure pancreatic juice obtained using this probe increased with time and reached 0.59 (0.14–1.23) arbitrary units (a.u.) after 30 min of incubation. The FIs of intestinal fluids and abdominal fluids with the chymotrypsin probe were close to zero: 0.02 a.u. (−0.04 to +0.17 a.u.) and 0.001 (−0.01 to +0.07 a.u.), respectively. The FI of drained fluids for the chymotrypsin probe 30 min after incubation showed positive correlations with amylase levels in corresponding fluid samples (Spearman’s rank-correlation test, ρ = 0.678, P < 0.001).
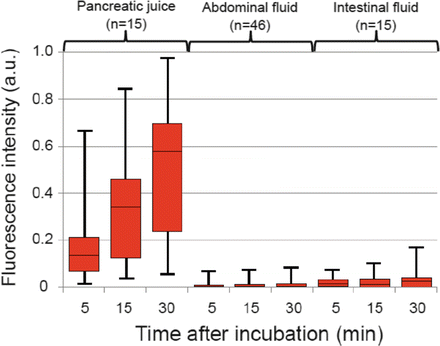

Fig. 36.2
Fluorescence intensity (FI) of drained fluid samples with the chymotrypsin probe according to the time after incubation. The FI increased with time in all fluid samples when the chymotrypsin probe yielded fluorescence almost exclusively in pure pancreatic juice (red bars). Median values (horizontal lines in boxes), interquartile range (box), and range (error bars) are shown in arbitrary units (a.u.)
Application of Chymotrypsin Probe for Intraoperative Identification of Pancreatic Leak and Prediction of Postoperative PF
To identify leakage of pancreatic juice during surgery, the chymotrypsin probe is sprayed on a filter paper that is attached on the cut surface of the pancreas for 10 s following resection of the pancreatic specimen (administration of this probe into a patient’s abdominal cavity has not yet been approved). Fluorescence images of leaking pancreatic juice on the pancreatic stump can be identified by naked-eye examination through light-blocking glasses (515 nm long-pass) under blue light illumination beginning 3 min after administration of the chymotrypsin probe (Fig. 36.3).
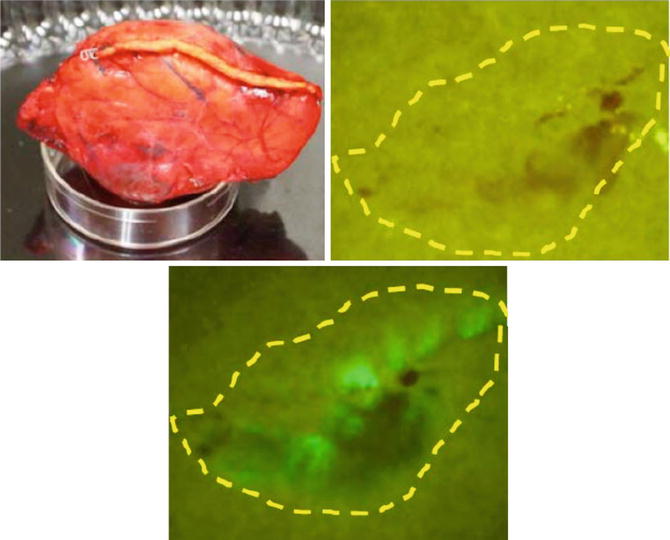

Fig. 36.3
Fluorescence imaging on a filter paper transcribing the pancreatic stump that had been closed with a surgical stapler during human distal pancreatectomy. Left. Gross appearance of the pancreatic stump in the resected specimen. Right. Appearance of the filter paper immediately after placement on the pancreatic stump (top). Fluorescence image of the filter paper obtained 1 min after spraying with the chymotrypsin probe (bottom). Yellow dotted line indicates the border of the pancreatic stump
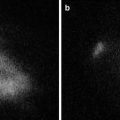
In our previous series consisting of 30 patients who underwent pancreatic resection [11], the fluorescence patterns of the pancreatic stump transcribed onto the filter paper were grossly classified into the following three types: fluorescent signal visualized only on the main pancreatic duct (MPD) stump (duct type, n = 16), fluorescent signal visualized on the MPD stump and the surrounding pancreatic parenchyma (diffuse type, n = 7), and no fluorescent signal (negative type, n = 7) (Fig. 36.4). Postoperatively, the incidence of PFs was 57 % in 23 patients with fluorescent signals on the pancreatic stump (duct type and diffuse type), whereas none of the patients with negative fluorescence signals suffered from postoperative PF. In addition, severe PF (ISGPF grade C, n = 3) developed only in patients with a diffuse-type fluorescence signal. These patients were deemed likely to be at high risk of postoperative PF because the lesion originated from the stump of the pancreatic parenchyma regardless of how accurately the pancreatojejunostomy was created. When FI of the pancreatic juice on filter paper was evaluated using the Maestro imaging system (CRi, Woburn, MA, USA), it correlated positively with the amylase levels in pancreatic juice collected from external pancreatostomy tubes on postoperative day 1 (ρ = 0.664, P < 0.001). These results suggest that the chymotrypsin probe during surgery enables detection of a pancreatic leak and estimation of the risk of postoperative PF. This knowledge may be helpful for surgeons regarding the need to undertake additional surgical procedures to close a pancreatic leak and to place (or omit) prophylactic abdominal drainage according to the risk of postoperative PF in each patient.
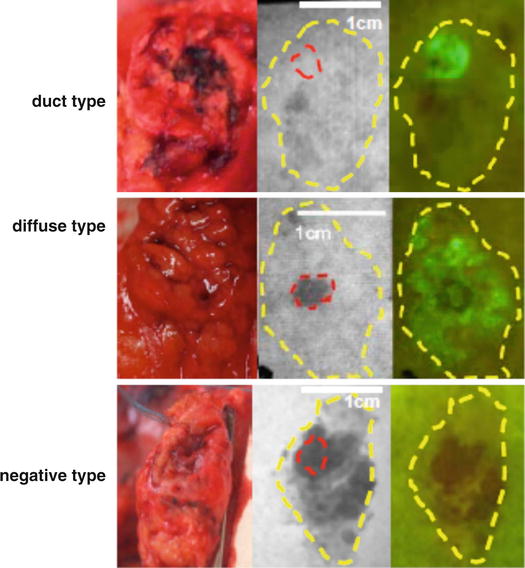

Fig. 36.4
Fluorescence patterns of the pancreatic stump transcribed onto filter papers. Left. Gross appearance of the pancreatic stump. Middle. Monochromatic appearance of the filter paper immediately after placement on the pancreatic stump. Right. Fluorescence images of the filter paper obtained 1 min after spraying with the chymotrypsin probe. Red and yellow dotted lines indicate the borders of the main pancreatic duct and pancreatic stump, respectively
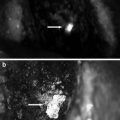
Preclinical Study for the Use of Chymotrypsin Probe in the Abdominal Cavity to Detect Pancreatic Leak During Surgery
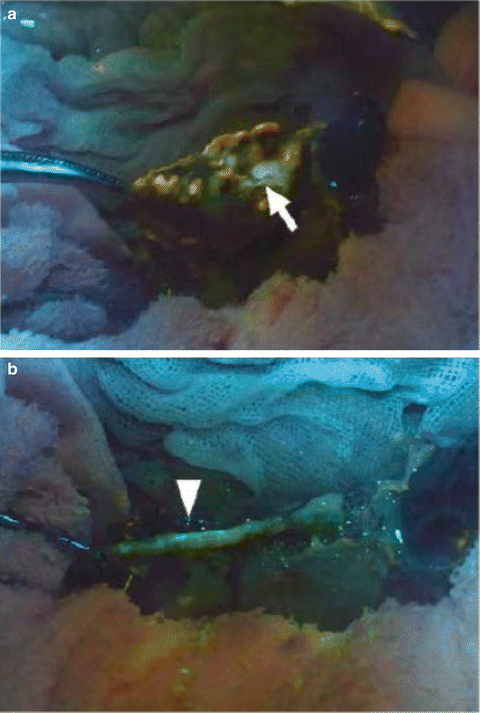
The authors believe that, in future, the chymotrypsin probe should be sprayed directly on the pancreatic stump in the patient’s abdominal cavity as it could enable surgeons to suture the point of the pancreatic leak with some accuracy. At present, this technique is being simulated using a swine model. In this model, the chymotrypsin probe is sprayed on the pancreatic stump within the abdominal cavity, following full mobilization and division of the pancreatic parenchyma with electrocautery or a surgical stapler. Fluorescence imaging using a FluorVivo imaging system (Indec, Santa Clara, CA, USA) with the blue-filter setting (excitation 470–490 nm; emission 515 nm long-pass) then enables identification of the pancreatic leak from the MPD following division of the pancreas with electrocautery (Fig. 36.5a) or from a stapler line following closure of the pancreas (Fig. 36.5b). Fluorescence of the leaking pancreatic juice is identifiable by naked-eye examination through light-blocking glasses (515 nm long-pass) beginning 1 min after administration of the chymotrypsin probe (see Video 36.1), which enables surgeons to make pinpoint sutures and confirm arrest of the pancreatic leak.