, Valery Kornienko1, Alexander Potapov2 and Igor Pronin3
(1)
Department of Neuroradiology, Burdenko Neurosurgery Intitute, Moscow, Russia
(2)
Department of Neurotrauma, Burdenko Neurosurgery Insitute, Moscow, Russia
(3)
Department of Neuroradiology, Burdenko Neurosurgery Institute, Moscow, Russia
Abstract
The problem of traumatic brain injury determines its social importance and search for new clinical methods of neurotrauma diagnosis and their introduction into clinical practice, as well as studying brain injury pathogenesis and prognosis of outcome. Chapter 1 reviews modern neuroimaging technologies and clarifies the main principles of modern diagnostic methods which allow better understanding of anatomical and pathophysiological changes in traumatic brain injury.
1.1 TBI-Related Social and Economic Problems
More than 1.2 million people die every year with about 20–50 million getting nonfatal trauma in traffic accidents (Global status report on road safety 2009). Brain damage is the cause of death in approximately 60 % of all trauma-related fatalities (Potapov et al. 2010).
According to World Health Organization data, countries with low and middle income show higher road accident fatality level (21.5 and 19.5 per 100,000), while countries with high income demonstrate a lower level (10.3 per 100,000). More than 90 % fatalities as a result of traffic accidents are reported in countries with low and middle income (Global status report on road safety 2009). The leading causes of death and disability of population in various age groups worldwide are summarized in Table 1.1.
Table 1.1
Leading causes of death by age worldwide, 2004 (shortened) (Global status report on road safety 2009)
Rank | 0–4 years | 5–14 years | 15–29 years | 30–44 years | 45–69 years | 70+ | Total |
|---|---|---|---|---|---|---|---|
1 | Perinatal causes | Lower respiratory infections | Road traffic injuries | HIV/AIDS | Ischemic heart disease | Ischemic heart disease | Ischemic heart disease |
2 | Lower respiratory infections | Road traffic injuries | HIV/AIDS | Tuberculosis | Cerebrovascular disease | Cerebrovascular disease | Cerebrovascular disease |
3 | Diarrheal diseases | Malaria | Tuberculosis | Road traffic injuries | HIV/AIDS | Chronic obstructive pulmonary disease | Lower respiratory infections |
4 | Malaria | Drownings | Violence | Ischemic heart disease | Tuberculosis | Lower respiratory infections | Perinatal causes |
5 | Measles | Meningitis | Self-inflicted injuries | Self-inflicted injuries | Chronic obstructive pulmonary disease | Trachea, bronchus, lung cancer | Chronic obstructive pulmonary disease |
In Russia 600,000 people suffer from TBI every year including 50,000 fatal cases with the number of disabled exceeding two million (Potapov et al. 2003). Traffic accidents, falls, assaults, etc., are the leading causes of different severity of brain trauma. TBI accounts for more than a half of trauma-related deaths, which is mostly common for traffic accidents (Teasdale et al. 1995; Marion et al. 1998).
By 2030, traffic accidents will be the 5th leading cause of death among all age groups worldwide (Global status report on road safety 2009) (Table 1.2).
Table 1.2
Leading causes of death, 2004 and 2030 compared (shortened) (Global status report on road safety 2009)
Total 2004 | Total 2030 | ||||
|---|---|---|---|---|---|
Rank | Leading cause | % | Rank | Leading cause | % |
1 | Ischemic heart disease | 12.2 | 1 | Ischemic heart disease | 14.2 |
2 | Cerebrovascular disease | 9.7 | 2 | Cerebrovascular disease | 12.1 |
3 | Lower respiratory infections | 7.0 | 3 | Chronic obstructive pulmonary disease | 8.6 |
4 | Chronic obstructive pulmonary disease | 5.1 | 4 | Lower respiratory infections | 3.8 |
5 | Diarrheal disease | 3.6 | 5 | Road traffic injuries | 3.6 |
6 | HIV/AIDS | 3.5 | 6 | Trachea, bronchus, lung cancer | 3.4 |
7 | Tuberculosis | 2.5 | 7 | Diabetes mellitus | 3.3 |
8 | Trachea, bronchus, lung cancer | 2.3 | 8 | Hypertensive heart disease | 2.1 |
9 | Road traffic injuries | 2.2 | 9 | Stomach cancer | 1.9 |
10 | Prematurity and low birth weight | 2.0 | 10 | HIV/AIDS | 1.8 |
Thus, motor vehicle-related neurotrauma and, first of all, head injury are the main causes of disability and mortality. The social significance of this problem makes us looking for new clinical methods of neurotrauma diagnosis and their introduction into clinical practice, as well as studying TBI pathogenesis and prognosis of outcome.
1.2 Neuroimaging in Assessment of Traumatic Brain Injury
In the 1970s, the introduction of computed tomography (CT) made it possible to visualize craniocerebral injury in vivo. Later appearance of magnetic resonance scanners with various sequences allowed better visualization of structural, metabolic, and functional aspects of the brain damage. Technological progress has made the basis for exponential improvement of the quality of imaging which is still ongoing. The beginning of the twenty-first century became the “golden” era for neuroimaging with its modern possibilities in studying structural and functional brain integrity, alongside with understanding brain functioning both in normal and pathological conditions.
When dealing with TBI, one should specify the mechanism of trauma and its extent and severity of cerebral and cranial damage. Timely identification of these factors allows prevention of numerous complications and irreversible changes. Different neuroimaging methods play the crucial role in the diagnostics of head injuries, their classification and extent, as well as distribution of patients for emergency surgery or intensive care. The recently developed CT and MRI modalities allow a better understanding of TBI pathophysiology, differentiating between primary and secondary brain damages. Primary brain injury occurs at the moment of direct impact, while secondary one evolves in minutes, hours, or days after the injury. Secondary factors can be prevented or treated depending on their timely and correct diagnosis, organization, and quality of the provided neurosurgical care (Gean 1994; Parizel et al. 2005; Potapov and Likhterman 2011).
1.3 Classifications of Traumatic Brain Injury
A widespread use of various imaging techniques has demonstrated that no universal method exists for studying different types of TBI and its consequences as well as evaluating a broad range of pathophysiological reactions of the brain at various posttraumatic periods. The clinical and morphological characteristics of the traumatic brain injury are used as the basis for development of the classification system for TBI. Attempts to classify TBI have been undertaken for a long time. In the pre-neuroimaging era, the specific emphasis was placed on their clinical manifestations, coma duration, posttraumatic amnesia, neurological and vegetative disorders, as well as on the results of postmortem studies of fatalities from TBI. The era of the computed tomography has permitted the development of classifications based on the lifetime morphological features of the brain injury. In particular, CT classifications were developed to identify various degrees of severity of focal contusions and diffuse injuries, intracranial hemorrhages, and hematomas (Gennarelli et al. 1982; Konovalov and Kornienko 1985; Marshall et al. 1991).
The following primary injuries are differentiated: focal contusions and lacerations, diffuse axonal injuries, primary brain stem injury, intracranial hemorrhages, etc. Secondary intracranial damages include delayed hematomas (epidural, subdural, intracerebral), cerebral blood flow and cerebrospinal fluid circulation disturbances as a result of subarachnoid or intraventricular hemorrhage, brain volume enlargement or brain swelling as a result of edema, hyperemia or venous congestion, intracranial hypertension, brain shift and herniation, etc. Secondary extracranial factors include arterial hypotension, hypoxemia, hypercapnia, anemia, etc. (Strich 1956; Gennarelli et al. 1982; Mendelow and Teasdale 1983; Povlishock 1986; Adams et al. 1989, 2000; Teasdale et al. 1995; Reilly and Bullock 2005; Potapov et al. 2011).
The following clinical forms of TBI can be identified: (1) brain concussion, (2) mild brain contusion, (3) moderate brain contusion, (4) severe brain contusion, (5) diffuse axonal injury, (6) brain compression, and (7) head compression.
An adequate staging and classification of TBI are obligatory conditions and the basis for studying pathological processes triggered by trauma, and developing effective methods for prevention and treatment of unfavorable consequences (Likhterman and Potapov 1998; Likhterman and Kasumova 2012; Potapov et al. 2011).
Smirnov (1949), the Russian morphologist and founder of the theory of the cerebral traumatic disease, defined it as a combination of etiology, pathological anatomy, pathophysiological mechanisms, its development, outcome, and complications.
1.4 CT and MRI in TBI
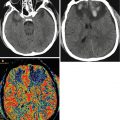
The advantages of CT scanning, as a method of choice for primary examination of patients with TBI, include prompt visualization of acute intracranial hemorrhages with their location sites, mass effect, and edema and identification of size and configuration of the ventricular system and subarachnoid spaces, bone fractures, or presence of foreign bodies, etc. Additional positive quality of this technique is its availability, speed of scanning, and compatibility with other life-support equipment (Parizel et al. 2005; Daviz et al. 2008; Kornienko and Pronin 2009). Therefore, CT has the ability of identifying urgent surgical situations, especially for patients with severe trauma.
In 1982, Gennarelli et al. developed a CT and clinically relevant classification of severe head injury and subdivided patients by focal and diffuse types of lesions in addition to categorizing them by Glasgow Coma Scale and coma duration.
The classification proposed by Marshall et al. (1991) was mainly based on the results of primary CT scans of patients with severe TBI, signs of midline shift, and mesencephalic cistern compression. It comprised a four-category scale for diagnosis of diffuse injury and two categories for diagnosis of mass lesions with special emphasis being placed on their possible surgical removal (Table 1.3).
Table 1.3
Diagnostic categories of types of abnormalities visualized on CT scanning (Marshall et al. 1991)
Category | Definition |
|---|---|
Diffuse injury I (no visible pathology) | No visible intracranial pathology seen on CT scan |
Diffuse injury II | Cisterns are present with midline shift 0–5 mm and/or lesion density present; no high-or mixed-density lesion > 25 cc May include bone fragments and foreign bodies |
Diffuse injury III (swelling) | Cisterns compressed or absent with midline shift 0–5 mm; no high-or mixed-density lesion > 25 cc |
Diffuse injury IV (shift) | Midline shift > 5 mm; no high-or mixed density lesion > 25 cc |
Evacuated mass lesion | All lesions surgically evacuated |
Non-evacuated mass lesion | High-or mixed-density lesion > 25 cc, not surgically evacuated |
This classification has proved to be helpful for creating the data bank and performing clinical studies of efficacy of various treatment methods. It also helped to determine a significant correlation between four diagnostic categories of diffuse injury and mortality rate, as well as ICP increase. However, CT scanning does not always help to predict outcome, because in severe brain trauma CT may fail to identify some pathological changes. Diagnostic possibilities and sensitivity of CT imaging in less severe brain injuries and in those of nonhemorrhagic nature are less significant. It was shown that intracranial pathology was detected in 5 % of patients with mild trauma (Glasgow Coma Scale score of 15) and in 30 % of cases with GCS score of 13 or less (Borg et al. 2004; Parizel et al. 2005). Despite the fact that clinical symptoms may predict pathological changes on CT scans, particularly in severe or moderate traumatic brain injury, it is not absolutely true for mild trauma, especially in children.
In addition, CT has a low sensitivity for detecting small cerebral damage foci in mild head injury and especially those adjacent to the cranial base and roof bones, as well as diffuse axonal injury and brain stem damages. CT scanning is also considered as a relatively insensitive method for detecting acute hypoxic and ischemic cerebral changes, subacute and chronic hemorrhages, and differentiating types of brain edema (Daviz et al. 2008; Kornienko and Pronin 2009).
MRI is more sensitive than CT in detecting brain damage in spite of its difficult application in the acute period of TBI, much time spent for scanning, and sedation of patients with motor and psychomotor agitation. MRI has serious contraindications for patients with unstable hemodynamics, and the presence of metal implants or cardiac pacemakers makes the examination impossible. There is a necessity for using special non-magnetic monitoring and ventilation equipment during scanning time in patients with severe TBI (Huisman et al. 2003, 2004; Parizel et al. 2005; Kornienko and Pronin 2009).
It is well known that MRI is more sensitive than CT in detecting nonhemorrhagic lesions and, in particular, DAI and other types of TBI during the subacute and chronic stages. Conventional MRI sequences of Т1, Т2, Т2-FLAIR, and Т2* gradient echo demonstrate different changes in the brain structures – mass effect, cistern compression, small intraparenchymal hemorrhages, and accumulation of blood in the subarachnoid space. Hemosiderin-sensitive 2D Т2* gradient echo is helpful in imaging of petechial, subacute, and chronic hemorrhages. Diffusion sequences improve detection of secondary acute infarctions in TBI. Up-to-date techniques are more sensitive to blood products (SWI, SWAN) and are useful in assessing cerebral perfusion (MR perfusion, ASL) and microstructural changes in the white matter tract integrity (diffusion-tensor MRI) and detecting brain activation areas (fMRI) (Gentry 1996; Sorensen et al. 1997; Liu et al. 1999; Huisman et al. 2003; Scheid et al. 2007; Daviz et al. 2008; Greenberg et al. 2009; Kornienko and Pronin 2009; Haacke et al. 2010).
1.4.1 Conventional MRI Sequences in Diagnosis of TBI
Т1-weighted imaging is used to study anatomy of the brain. Processes that shorten Т1 relaxation time result in the increased MR signal on Т1 images, as, for instance, in hemorrhages with methemoglobin.
Т2-weighted imaging is used to detect pathology with high water content in tissues, especially with edematous tissues, and is sensitive to deoxyhemoglobin and hemosiderin.
Т2-FLAIR is described as a sequence of suppressed MR signal from the CSF and accentuated pathology revealed on Т2 FSE sequences, especially with the abnormality being located in the cortical and periventricular regions, as well as diffuse axonal injury (Haacke et al. 2010). This impulse sequence also allows an accurate visualization of acute subarachnoid hemorrhages and has an equal or even higher sensitivity than CT (Campball and Zimmerman 1998; Parizel et al. 2005).
Т2* gradient echo sequences are used for detection of small hemorrhages because of their high sensitivity to magnetic susceptibility effects. At the same time, small and petechial hemorrhages may be identified in the acute posttraumatic period, as well as in months and even years after trauma, when gradient echo sequences allow visualization of hypointense hemosiderin deposits after diffuse axonal injury (Parizel et al. 1998, 2001, 2005, Lin et al. 2001; Scheid et al. 2003).
1.4.2 Advanced MRI Sequences in Diagnosis of TBI
SWI (susceptibility-weighted imaging) and SWAN (T2 star-weighted angiography) are modern Т2* sequence modifications and actually are 3D gradient echo with a high spatial resolution and flow compensation; they are especially sensitive to blood products in hemorrhages and deoxyhemoglobin in the venous blood (Reichenbach et al. 1997; Haacke et al. 2010; Pronin et al. 2011). SWI has proved to be more sensitive than 2D Т2* GRE in diffuse axonal injury (Tong et al. 2004; Babikian et al. 2005; Haacke et al. 2010).
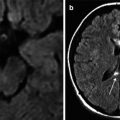
DWI – diffusion-weighted imaging – is sensitive to the motion of water molecules (hydrogen protons) in tissues; it allows a better understanding of brain physiology, with some of its aspects being impossible to study when using other sequences. DWI and apparent diffusion coefficient (ADC) obtained on maps are used in differential diagnosis of cytotoxic and vasogenic edema as a result of brain injury or ischemia (Kawamata et al. 2000; Huisman et al. 2003; Haacke et al. 2010). ADC reduction suggests presence of a cytotoxic (intracellular) edema, while its increase indicates development of a vasogenic (extracellular) edema. DWI plays the crucial role in detection of acute stroke and diffuse axonal injury (Liu et al. 1999; Haacke et al. 2010).
1H MR spectroscopy allows identification of various metabolites in the brain in vivo reflecting changes in both biochemical processes and structural damage of cells. Brain injury induces changes in N-acetylaspartate (NAA) (a neuronal biomarker), the decrease of which in pathology foci is indicative of its primary damage, while decrease in NAA in normally appearing brain areas may be indicative of DAI and Wallerian degeneration (Cecil et al. 1998a, b; Friedman et al. 1999; Brooks et al. 2000; Garnett et al. 2000, 2001; Portella et al. 2000; Yoon et al. 2005; Babikian et al. 2006; Holshouser et al. 2006; Shutter et al. 2006; Haacke et al. 2010). An increase in choline (Cho) levels (a marker for membrane disruption, synthesis, or repair) can be detected in the white matter and is associated with myelin damage (Ross et al. 1998; Haacke et al. 2010). Presence of lactate (Lac) (a result of anaerobic glycolysis) is indicative of a hypoxic/ischemic injury. Currently, two methods are used in proton MR spectroscopy – single- and multi-voxel modes. In single-voxel MR spectroscopy, only one region (voxel) of the brain is selected for the analysis. By analyzing frequencies of the signal registered from the voxel, the distribution of metabolite peaks is obtained by the chemical shift scale. In multi-voxel MR spectroscopy, MR spectra are obtained for several voxels simultaneously. That allows comparing spectra of different areas in the studied zone, as well as obtaining parametric maps. The concentration of particular metabolites on these maps is marked by color, and thus it is possible to visualize the metabolite distribution in the brain (Kornienko and Pronin 2009).
1.4.3 MRI Classification of TBI
Based on the use of routine Т1 and Т2 MRI sequences and analysis of the data obtained from patients in the acute period of severe TBI, Firsching et al. (2001) proposed their classification of severe TBI (Table 1.4). According to the authors, an accurate location of lesion foci in the brain stem (primary or secondary) plays the crucial role in prognosis of TBI outcome.
Diagnostic groups of lesions based on MRI after severe head injury | |
|---|---|
Supratentorial injuries only | Grade I |
Brain stem lesions with or without supratentorial lesions | Grade II, unilateral lesion of the brain stem at any levels ± I |
Grade III, bilateral lesion of the mesencephalon ± I–II | |
Grade IV, bilateral pontine lesion ± I–III | |
At the same time, classification by Firsching et al. (2001) of the brain stem damage level was based on the results of routine MRI sequences and did not consider the mechanism of brain trauma and its severity and localization of supratentorial injuries. Mannion et al. (2007) studied 46 patients with acute severe TBI by using the following sequences: Т2 MRI, proton density, FLAIR, and gradient echo imaging. The authors classified all patients into three groups based on the mechanism of brain stem injury: (1) brain stem lesions caused by DAI, (2) brain stem lesions as a result of supratentorial herniation, and (3) isolated brain stem lesions with small cortical contusions. It was shown that all patients in first and second groups showed unfavorable outcome in 6 months after trauma, while only two patients with isolated brain stem lesions had favorable outcome. Of 33 patients with supratentorial lesions, only 18 had unfavorable outcome. Despite an evidently significant relationship existing between brain stem lesions and unfavorable outcome, the authors have concluded that brain stem injury is not an absolute indicator of unfavorable outcomes. The role of MRI scanning in assessing the prognosis after severe and moderate TBI was also analyzed in studies of Kornienko and Pronin (2009), Lagares et al. (2009), and Hilario et al. (2012).
1.4.4 Other Neuroimaging Methods
SPECT, PET, xenon-enhanced CT, and fMRI are used in clinical practice when assessing cognitive and neuropsychological changes in patients with traumatic brain injury (Jacobs et al. 1994, 1996; Abdel-Dayem et al. 1998; Berrouschot et al. 1998; Weckesser and Schober 1999; Hofman et al. 2001; Warwick 2004; Devous 2005; Daviz et al. 2008). These methods allow a qualitative assessment of cerebral perfusion that cannot be established with routine CT and MRI studies. The single-photon emission computer tomography (SPECT) reflects the brain perfusion only. Unlike SPECT, positron emission tomography (PET) has higher spatial resolution capabilities, which allows mapping of metabolism parameters in addition to the quantitative evaluation of the blood flow (Cikrit et al. 1997, 1999; Buttler et al. 1998; Wintermark et al. 2001a, b; Chen et al. 2004; Wu et al. 2004; Vespa et al. 2005; Wintermark et al. 2005; Catala-Temprano et al. 2007; Haacke et al. 2010). SPECT and PET in combination with CT or MRI are the most perspective ones in this regard. However, the main drawbacks of PET and SPECT are the necessity of using radiotracer, high cost, and insufficient availability in clinical settings.
Functional MRI (fMRI) is described as brain activation mapping based on hemodynamic response in the increased neuronal cortical activity as a response to the special motor, sensor, and other stimulation tasks (Kornienko and Pronin 2009). This method is used for studying various groups of patients with brain injury, yet it has not been introduced into routine clinical examinations of TBI. Functional MRI uses an echo-planar imaging approach and evaluates changes in the BOLD (blood oxygen level dependent) signal (Hattori et al. 2004; Strangman et al. 2005; Haacke et al. 2010). An increase in neuronal activity results in increase of blood flow, thus raising the local blood oxygenation levels (McAllister et al. 1999, 2006; Logothesis et al. 2001; Karunanayaka et al. 2007; Newsome et al. 2008; Strangman et al. 2009; Haacke et al. 2010). Functional MRI signal in brain injury can be modified as a result of change or combinations of changes of neuronal activity as well as regulation of the blood flow. Patients who are unable to perform special tests and tasks during fMRI cannot take part in these studies. The resting-state fMRI provides a new opportunity to study spontaneous fluctuations in the BOLD signal in TBI patients. This approach is an option to study patients with TBI of different severity ranging from concussion to vegetative state (Greicius et al. 2003; Nagai et al. 2004; Fox et al. 2005; Fox and Raichle 2007; Owen et al. 2006; Vincent et al. 2007; Haacke et al. 2010). However, the need for immobilization and sedation of patients with psychomotor agitation, seizures, or comatose state restricts the use of fMRI in TBI.
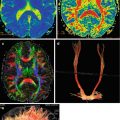
1.5 Diffusion-Tensor MRI and MR Tractography
Diffuse axonal injury (DAI) is one of the leading causes of morbidity and mortality in TBI patients. The anatomical structures that are most often injured in DAI are subcortical white matter, corpus callosum, dorsolateral regions of the midbrain, and subcortical formations (Strich 1956; Adams et al. 1982; Gennarelli et al. 1982, 1986; Gentry et al. 1988; Gean 1994; Murray et al. 1996; Povlishock and Stone 2001; Reilly and Bullock 2005).
Microscopic morphological studies demonstrated axonal damage in the form of axonal retraction balls already in 12 h following trauma. These balls are visible when being stained with silver impregnation (Kasumova 1998; Kasumova 1991) or by immunocytochemical method (Gentleman et al. 1993; Sheriff et al. 1994). In addition to the partial axonal damage, their subsequent condition depends on the degree of expression of secondary reactions – hemorrhages, edema, local perfusion changes, and various cascades of biochemical reactions (Gennarelli et al. 1986; Adams et al. 1989; Maxwell et al. 1997; Povlishock and Katz 2005).
Until recently, there has been no accurate method for in vivo diagnosis of prevalence and severity of DAI because CT and routine MRI poorly differentiate this type of injuries (Gentry et al. 1988; Kelly et al. 1988). CT findings characterized by petechial hemorrhages in the corpus callosum, white gray matter junction, and brain stem, more often the midbrain, could be seen only in 10 % of patients with severe DAI (Blumbergs et al. 1994, 1995; Xu et al. 2007). Delayed CT scans are often relatively normal or characterized by diffuse brain atrophy with the enlargement of ventricles and subarachnoid spaces (Whyte and Rosental 1993; Diaz-Marchan et al. 1996). Thus, more sensitive diagnostic methods should be found and used for diffuse axonal injury.
MRI, especially Т2-FLAIR and Т2* gradient echo, is much more sensitive for the accurate diagnosis and prognosis of TBI outcome depending on the level of hemispheric and brain stem damage (Firsching et al. 2001; Mannion et al. 2007). It was shown that the diffusion-weighted MRI (DWI) could reveal damages not visible even on Т2, Т2*, and Т2-FLAIR MRI (Huisman et al. 2003).
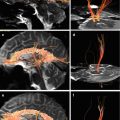
An application of diffusion-tensor MRI (DT MRI) as well as the retrieval of 3D images has opened up new possibilities for the qualitative and quantitative assessment of brain pathway damage. Thus, in a clinical setting, in vivo, visualization of DAI severity can be obtained (Liu et al. 1999; Arfanakis et al. 2002; Huisman et al. 2004; Wilde et al. 2006; Benson et al. 2007; Kim et al. 2008; Zakharova et al. 2007, 2008, 2009, 2010a, b; Zakharova 2013).
DTI evaluates diffusion characteristics of water molecules in the region of interest as well as directional dependence of water diffusion (anisotropy) and, thus, provides information about the degree of white matter tract integrity (Melhem et al. 2000; Prefferbaum et al. 2000; Papadakis et al. 2002; Pronin et al. 2008). Diffusion anisotropy in different regions of the white matter is not uniform; it reveals differences in myelinization of fiber tracts, their diameter and orientation (Pierpaoli et al. 1996). Pathological processes that modify the white matter microstructure such as fiber disruption and separation, breakdown of myelin, neuronal swelling, and an increase or decrease of extracellular space have an impact on diffusion and anisotropy parameters (Ducreux et al. 2005; Povlishock and Katz 2005; Wilde et al. 2006; Grinberg et al. 2011).
The most commonly used quantitative values in the assessment of DWI and DTI are the apparent diffusion coefficient (ADC) and fractional anisotropy (FA), respectively (Basser and Pierpaoli 1996; Basser and Pierpaoli 1998). DTI studies in patients with head injuries have shown that FA is reduced in the first week after injury, despite of no changes in the white matter observed on CT or routine MRI (Arfanakis et al. 2002; Huisman et al. 2004). Reduced FA in cases with DAI was found in the anterior and posterior regions of the corpus callosum, posterior limb of the internal capsule and in the external capsule. Similar data were obtained in a study with a whole-brain white matter analysis of DTI data (Inglese et al. 2005; Benson et al. 2007).
It has been found that changes in diffusion-tensor parameters reflect degeneration of axons and myelin sheath leading to their atrophy. This process takes months and even years following DAI (van der Knaap 2005; Xu et al. 2007; Rutgers et al. 2008; Sidaros et al. 2008).
Individual observations of structural degeneration of corpus callosum fibers and fornix conducted at different time intervals following head injuries as well as varying severity have been described and visualized by MR tractography (Naganawa et al. 2004; Voss et al. 2006; Sugiyama et al. 2009). The commissural and projection pathways were chosen for a three-dimensional reconstruction in publications of various authors. It was based on the fact that the corpus callosum was easily distinguished with DTI on the midsagittal level by using colored fractional anisotropy mapping, while corticospinal tracts had a distinct unidirectionality. Other fiber tracts were more difficult to isolate especially on condition of high frequency and reproduction (Naganawa et al. 2004; Xu et al. 2007).
1.6 Cerebral Blood Flow Assessment
Brain perfusion disorders are the most common pathophysiological phenomena in TBI that is why studying cerebral hemodynamics is essential both for understanding pathogenic mechanisms of the traumatic brain disease and for developing treatment tactics and prognostic models (Meier and Zierler 1954; Contoni 1960; Fisher 1961; Reivich et al. 1961; Nariai et al. 1995, 1998; Mirzai and Saami 2000; Derdeyn et al. 2002). Research of rCBF disturbances became possible with the development of noninvasive methods of study, including positron emission tomography (PET), single-photon emission computer tomography (SPECT) (Madeau et al. 1995; Alexandrov et al. 1996; Launes et al. 1997; Davalos and Bennett 2002), xenon-enhanced CT (XeCT) (Ritter et al. 1999; Wintermark et al. 2001a), arterial spin labeling method (ASL) (Deibler et al. 2008a, b, c), as well as CT perfusion, MR perfusion (dynamic susceptibility contrast, DSC), and other methods (Shakhnovich and Shakhnovich 1996; Asenbaum and Baumgartner 2001; Garnett et al. 2001; Wintermark et al. 2005). CT perfusion method is the most available technique which is routinely used in diagnosis of acute stroke (Wintermark et al. 2008; Haacke et al. 2010), as well as in studying neoangiogenesis in patients with brain tumors (Covarrubias et al. 2004; Pronin et al. 2005; Kornienko and Pronin 2009; Haacke et al. 2010).
Xenon is a stable isotope and as a contrast material is easily diffused across the intact blood-brain barrier (Latchaw et al. 1987, 2003; Good et al. 1992). Xenon-enhanced CT is more commonly used in cerebrovascular diseases and rarely in TBI (Ritter et al. 1999; Wintermark et al. 2001b). This method provides an accurate quantitative cerebral blood flow evaluation, multilevel examination of the brain with an option of a 10 min interval examination repeat. The disadvantages of this method include a relatively long period of scanning and sensitivity to motion artifacts (Wintermark et al. 2005).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree