In contrast with the previous criteria published in 1984 by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association, the new criteria proposed in 2007 incorporated in the diagnostic framework the use of biomarkers that are able to assess the underlying pathophysiologic mechanism. The combination of clinical and biologic approaches makes a diagnosis of Alzheimer’s disease possible before the dementia stage. The core clinical criteria continue to be the cornerstone of the diagnosis in clinical practice, but biomarker evidence is expected to enhance the specificity for the diagnosis of Alzheimer’s disease.
New concepts for the clinical definition of Alzheimer’s disease
For more than 25 years, the diagnosis of Alzheimer’s disease (AD) has been based on the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer’s Disease and Related Disorders Association (ADRDA) criteria, according to which the diagnosis is classified as definite (clinical diagnosis with histologic confirmation), probable (typical clinical syndrome without histologic confirmation), or possible (atypical clinical features but no alternative diagnosis apparent; no histologic confirmation). According to this definition, clinicians used the term AD to refer to a clinical dementia entity that typically presents with a characteristic progressive amnestic disorder with the subsequent appearance of other cognitive and neuropsychiatric changes that impair social function and activities of daily living. In the NINCDS-ADRDA criteria, biological investigation (blood and cerebrospinal fluid [CSF]) and neuroimaging examination (computed tomography [CT] scan or magnetic resonance [MR] imaging) were only proposed to exclude other causes of the dementia syndrome (eg, vascular lesions, tumors, infectious or inflammatory processes). Typical sensitivity and specificity values for the diagnosis of probable AD with the use of NINCDS-ADRDA criteria are 81% and 73%, respectively.
The recent advances in biomarkers of AD, which provide in vivo information about the pathophysiologic process associated with AD, have stimulated the proposal of new diagnostic criteria by the International Working Group (IWG) for New Research Criteria for the Diagnosis of AD. According to this framework, the diagnosis of AD was reconceptualized as a clinical-biological entity with a specific clinical phenotype and confirmatory in vivo pathophysiologic evidence of AD. This combined clinical and biological approach may improve the accuracy of the diagnosis. Because this new diagnostic framework no longer refers to dementia, it permits a clinical diagnosis to be established at an early prodromal/predementia stage of the disease that was previously incorporated in the heterogeneous concept of mild cognitive impairment (MCI).
More recently, the National Institute of Aging-Alzheimer’s Association (NIA-AA) workgroups published new diagnostic guidelines for AD that also incorporate biological and imaging markers to establish an earlier diagnosis of AD.
In both diagnostic criteria, a consideration of preclinical stages of AD is proposed, according to which the pathophysiologic process of the disease precedes the clinical manifestations. Because this condition has been studied, but there are no clinical implications at this time, this aspect of AD is not discussed in this article.
Identification of the clinical symptoms of AD at an early stage
Progression of Cognitive Symptoms Follows the Progression of the Underlying Cerebral Lesions
The most prominent feature of AD is a decline in cognitive function. In the early stages of AD, critical areas for episodic memory are already affected by neuropathologic changes (neurofibrillary degeneration) in medial temporal regions (hippocampal formations, parahippocampal gyrus, and entorhinal cortex). As a consequence, episodic memory deficit is an initial and reliable neuropsychological marker of AD. Memory impairment of recent events, unusual repeated omissions, and difficulty in learning new information characterize the first clinical signs. As the disease progresses, the clinical symptoms may involve language disorders, visuospatial and recognition deficits, and difficulties in executing more complex tasks of daily living, leading to dementia. The progression of cognitive deficits is consistent with the extension of underlying pathologic lesions (more specifically, of tau lesions) through the neocortical associative areas, as established by Braak and Braak.
Amnesic Syndrome of the Medial Temporal Type as a Marker of Hippocampal Damage
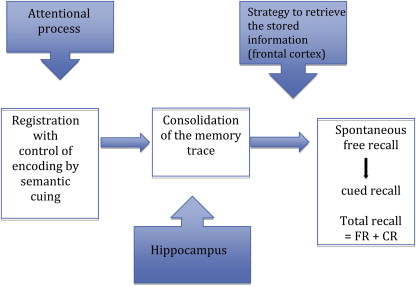
A limit for establishing an early AD diagnosis concerns the ability to identify the specific pattern of memory disorders in relation to damage to the hippocampal formations that characterize the disease and to distinguish them from age-related attention disorders, or from retrieval deficits that are seen in depression, frontal lobe dysfunction, subcortical dementia, or some vascular dementias. The neuropsychological testing, when adequate memory tests are used, can quantify and qualify the memory deficit and can therefore distinguish genuine memory impairment (eg, failure of information storage and new memory formation) from attention or retrieval disorders (such as normal aging or frontal disorders) ( Fig. 1 ). More particularly, test paradigms that provide encoding specificity are of great interest and improve the diagnostic accuracy. Within such memory paradigms, test materials are encoded along with specific cues (eg, semantic cues) that are used to control for an effective encoding and are subsequently presented to maximize retrieval. Memory tests that coordinate encoding and retrieval processes include the Free and Cued Selective Reminding Test (FCSRT) or similar cued recall paradigms. The FCSRT can identify the amnesic syndrome of the medial temporal type (also called the hippocampal type) observed in AD, defined by (1) poor free recall (as in any memory disorders) and (2) decreased total recall caused by an insufficient effect of cueing. The low performance of total recall despite retrieval facilitation indicates poor storage of information. Measures of sensitivity to semantic cueing can successfully differentiate patients with AD from healthy controls, even when patients are matched to controls on their Mini-Mental State Examination (MMSE) scores or when disease severity is mild. By isolating patients with an amnesic syndrome of the hippocampal type among those with MCI, the FCSRT is able to distinguish patients at an early stage of AD from MCI nonconverters with high sensitivity (80%) and specificity (90%).
In an AD population, a recent MR imaging study showed that the performance of the FCSRT was correlated with the left medial temporal lobe volume assessed both by voxel-based morphometry analysis and the automatic volumetric method, reinforcing the idea that the measure of episodic memory by this test may be considered a useful clinical marker of medial temporal damage. These correlations within the hippocampus were specially localized in the CA1 field, a region known to be involved in memory storage, and to be affected early by AD neurobiological processes.
The amnestic syndrome of the medial temporal type differs from functional and subcorticofrontal memory disorders, which are characterized by a low free recall performance with a normalization (or a quasinormalization) of the performance in total recall because of good efficacy of cueing. This subcortical-frontal profile of memory impairment is observed in depression, vascular dementia, frontotemporal dementia, and subcortical dementia, showing its additional value for differential diagnosis.
Neuropsychological tests should also assess other cognitive functions that may be affected even at a mild stage of the disease, such as executive functions, visuospatial capacities, language, or semantic knowledge.
Severity of Disease
Different stages of severity are described in AD, from mild to moderate and severe dementia. In the recent AD criteria, the terms prodromal AD, predementia AD, or AD at the stage of MCI (MCI caused by AD) were proposed in reference to the early stage of the disease.
The MMSE assesses global cognitive efficiency and it is generally used to evaluate dementia severity. Although MMSE is not a specific neuropsychological test for AD diagnosis, it is easy and quick to administer and can track the overall progression of cognitive decline. Longitudinal studies have shown that the mean annual rate of progression of cognitive impairment using MMSE is approximately 2 to 6 points. The Clinical Dementia Rating Scale (CDR), based on an overall evaluation of the patient’s condition, offers incremental stages of severity. Functional decline increases with disease progression. In the MCI stage, the patient can live alone. In mild stages of AD, patients require limited home care. In moderate stages, patients need supervision and regular assistance in most activities. In severe stages, residential health care may be required.
Identification of the clinical symptoms of AD at an early stage
Progression of Cognitive Symptoms Follows the Progression of the Underlying Cerebral Lesions
The most prominent feature of AD is a decline in cognitive function. In the early stages of AD, critical areas for episodic memory are already affected by neuropathologic changes (neurofibrillary degeneration) in medial temporal regions (hippocampal formations, parahippocampal gyrus, and entorhinal cortex). As a consequence, episodic memory deficit is an initial and reliable neuropsychological marker of AD. Memory impairment of recent events, unusual repeated omissions, and difficulty in learning new information characterize the first clinical signs. As the disease progresses, the clinical symptoms may involve language disorders, visuospatial and recognition deficits, and difficulties in executing more complex tasks of daily living, leading to dementia. The progression of cognitive deficits is consistent with the extension of underlying pathologic lesions (more specifically, of tau lesions) through the neocortical associative areas, as established by Braak and Braak.
Amnesic Syndrome of the Medial Temporal Type as a Marker of Hippocampal Damage
A limit for establishing an early AD diagnosis concerns the ability to identify the specific pattern of memory disorders in relation to damage to the hippocampal formations that characterize the disease and to distinguish them from age-related attention disorders, or from retrieval deficits that are seen in depression, frontal lobe dysfunction, subcortical dementia, or some vascular dementias. The neuropsychological testing, when adequate memory tests are used, can quantify and qualify the memory deficit and can therefore distinguish genuine memory impairment (eg, failure of information storage and new memory formation) from attention or retrieval disorders (such as normal aging or frontal disorders) ( Fig. 1 ). More particularly, test paradigms that provide encoding specificity are of great interest and improve the diagnostic accuracy. Within such memory paradigms, test materials are encoded along with specific cues (eg, semantic cues) that are used to control for an effective encoding and are subsequently presented to maximize retrieval. Memory tests that coordinate encoding and retrieval processes include the Free and Cued Selective Reminding Test (FCSRT) or similar cued recall paradigms. The FCSRT can identify the amnesic syndrome of the medial temporal type (also called the hippocampal type) observed in AD, defined by (1) poor free recall (as in any memory disorders) and (2) decreased total recall caused by an insufficient effect of cueing. The low performance of total recall despite retrieval facilitation indicates poor storage of information. Measures of sensitivity to semantic cueing can successfully differentiate patients with AD from healthy controls, even when patients are matched to controls on their Mini-Mental State Examination (MMSE) scores or when disease severity is mild. By isolating patients with an amnesic syndrome of the hippocampal type among those with MCI, the FCSRT is able to distinguish patients at an early stage of AD from MCI nonconverters with high sensitivity (80%) and specificity (90%).
In an AD population, a recent MR imaging study showed that the performance of the FCSRT was correlated with the left medial temporal lobe volume assessed both by voxel-based morphometry analysis and the automatic volumetric method, reinforcing the idea that the measure of episodic memory by this test may be considered a useful clinical marker of medial temporal damage. These correlations within the hippocampus were specially localized in the CA1 field, a region known to be involved in memory storage, and to be affected early by AD neurobiological processes.
The amnestic syndrome of the medial temporal type differs from functional and subcorticofrontal memory disorders, which are characterized by a low free recall performance with a normalization (or a quasinormalization) of the performance in total recall because of good efficacy of cueing. This subcortical-frontal profile of memory impairment is observed in depression, vascular dementia, frontotemporal dementia, and subcortical dementia, showing its additional value for differential diagnosis.
Neuropsychological tests should also assess other cognitive functions that may be affected even at a mild stage of the disease, such as executive functions, visuospatial capacities, language, or semantic knowledge.
Severity of Disease
Different stages of severity are described in AD, from mild to moderate and severe dementia. In the recent AD criteria, the terms prodromal AD, predementia AD, or AD at the stage of MCI (MCI caused by AD) were proposed in reference to the early stage of the disease.
The MMSE assesses global cognitive efficiency and it is generally used to evaluate dementia severity. Although MMSE is not a specific neuropsychological test for AD diagnosis, it is easy and quick to administer and can track the overall progression of cognitive decline. Longitudinal studies have shown that the mean annual rate of progression of cognitive impairment using MMSE is approximately 2 to 6 points. The Clinical Dementia Rating Scale (CDR), based on an overall evaluation of the patient’s condition, offers incremental stages of severity. Functional decline increases with disease progression. In the MCI stage, the patient can live alone. In mild stages of AD, patients require limited home care. In moderate stages, patients need supervision and regular assistance in most activities. In severe stages, residential health care may be required.
Biomarkers of AD
The term biomarkers refers to “an objective measure of a biological or pathogenic process that can be used to evaluate disease risk or prognosis, to guide clinical diagnosis or to monitor therapeutic interventions.” These biomarkers include both neuroimaging and biological tools.
Structural Imaging Based on MR Imaging: Atrophy of Medial Temporal Structures as a Topographic and Neurodegenerative Marker
For many years, the use of CT and MR imaging in the evaluation of AD has been proposed for excluding neurosurgical lesions, such as brain tumors or subdural hematomas, or cerebrovascular lesions (cerebral infarcts, white matter lesions, microbleeds) that may account for vascular dementia. Modern neuroimaging extends beyond this traditional role of excluding other conditions and MR imaging is now considered an essential part of AD diagnosis.
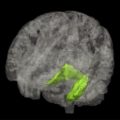
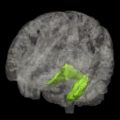
The volume of the hippocampus is significantly reduced in AD compared with age-matched control subjects, by 30% to 40% in moderate AD, 15% to 30% in mild AD (MMSE >20), and about 10% to 12% in early AD (MMSE about 27). Atrophy of medial temporal structures detected by high-resolution MR imaging is considered to be a reliable diagnostic marker at the MCI stage, and supports the diagnosis of AD. A recent meta-analysis estimated that medial temporal atrophy has 73% sensitivity and 81% specificity for predicting whether patients with amnestic MCI will convert to dementia. In the more advanced stage of AD, atrophy in temporal, parietal, and frontal neocortices is associated with language, praxic, visuospatial, and behavioral impairments.
Cortical atrophy, especially hippocampal atrophy, assessed by MR imaging is considered a topographic biomarker. Neuropathologic studies in patients with AD showed that the hippocampal volume measured in vivo by MR imaging correlates with tau deposition, Braak stage, and neuronal counts. Moreover, atrophy of medial temporal structures was correlated with memory deficit.
However, medial temporal atrophy is not specific enough to serve as an absolute criterion for the clinical diagnosis of AD at the MCI stage. A decreased volume of the hippocampus can be observed in neurodegenerative conditions other than AD, even in depression and normal aging. The overlap of hippocampal volume measures between AD and normal aging limits its interpretation when considered without clinical data.
To facilitate clinical investigation, several rating scales have been developed to quantify the degree of medial temporal lobe atrophy by visual inspection of coronal T1-weighted MR imaging. Visual rating scales provide 80% to 85% sensitivity and specificity to distinguish patients with AD from those with no cognitive impairment. These scales are widely used and can predict the risk of conversion to dementia in the MCI population. New automated methods of segmentation are also valuable tools for measuring hippocampal volume and may be useful in clinical practice in the future.
The combination of other markers (such as CSF biomarkers) with measures of hippocampal volume increases the accuracy of a diagnosis of early AD. However, rates of change in several structural measures, including whole brain, entorhinal cortex, hippocampal, and temporal lobe volumes, as well as ventricular enlargement, correlate closely with changes in cognitive performance, supporting their validity as markers of disease progression.
Single-Photon Emission CT and Fluorodeoxyglucose Positron Emission Tomography as a Marker of Neuronal Dysfunction
Functional neuroimaging techniques include measurement of blood flow ( 99m Tc-hexamethylpropyleneamine oxime [HMPAO] or 133 Xe) with single-photon emission CT (SPECT), and positron emission tomography (PET).
SPECT has the advantage of greater availability than PET imaging but PET provides images with higher resolution. 99m Tc-HMPAO SPECT is a useful neuroimaging technique for distinguishing AD from frontotemporal dementia (FTD) but a systematic review reported a clinical accuracy for patients with AD versus control individuals of only 74%. However, recent work in a group with amnestic MCI showed that an automated quantitative tool for brain perfusion SPECT images using the mean activity in right and left parietal cortex and hippocampus was able to distinguish patients at an early stage of AD from patients with stable MCI (sensitivity, specificity, and accuracy of 82%, 90%, and 89%, respectively).
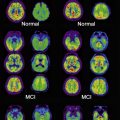
PET with fluorodeoxyglucose (FDG) to measure glucose metabolism has shown good accuracy in distinguishing patients with AD, even at an early stage, from both normal control individuals and patients with non-AD dementias. This imaging method has been approved in the United States for diagnostic purposes. A meta-analysis has reported a sensitivity and specificity of 86% for the diagnosis of AD, although there were wide variations between studies. A reduction of glucose metabolism in bilateral temporal parietal regions and in the posterior cingulate cortex is the most common finding in AD.
CSF Amyloid and Tau Levels as Pathophysiologic Markers
The challenges for establishing an early diagnosis and for the development of disease-modifying drugs have created a need for biomarkers that reflect core pathologic elements of the disease. The 2 core pathologic hallmarks of AD are (1) amyloid plaques, mainly composed of a heart of aggregated β-amyloid (Abeta) protein; and (2) neurofibrillary tangles (NFT), composed of abnormally hyperphosphorylated forms of the tau protein. In AD, the biomarkers that have been developed reflect amyloid and neurofibrillary tangle abnormalities. Because CSF is in direct contact with the extracellular space of the brain, the CSF is the optimal source of biological physiopathologic biomarkers. The CSF levels of total tau (T-tau), phosphorylated tau (P-tau), and β-amyloid peptide 1-42 (Ab) can distinguish controls from individuals with AD, with a sensitivity and specificity between 80% and 90% even in the early stages of the disease. In autopsy-proven AD, the P-tau/Ab ratio has the best sensitivity (91.6%) and specificity (85.7%) for differentiating AD from normal aging. The combination of low Ab and high levels of T-tau and P-tau, or, more specifically, the abnormal ratio of Ab to P-Tau, are associated with high rates of progression from amnestic MCI to AD dementia with a sensitivity of 95% and a specificity of 87%.
Neuropathologic studies that analyzed correlations between the levels of in vivo CSF biomarkers with the intensity of the postmortem cerebral lesions found correlations between CSF Ab with amyloid plaque load and between CSF T-tau and P-tau with neurofibrillary tangles. In a recent work using the new IWG criteria, high CSF T-tau and P-tau, but not CSF Ab, were correlated with hippocampal atrophy, suggesting that CSF tau markers are related to the neuronal loss associated with AD.
The combined analysis of the CSF biomarkers, specially the ratio P-tau/Ab, is also helpful for the differential diagnosis between AD and frontotemporal lobar degeneration (FTLD), whatever its behavioral presentation (FTD) or semantic dementia. CSF biomarkers are able to distinguish FTLD with a sensitivity and specificity of around 90%. These results are similar to those from a previous study of patients with FTD shown at autopsy or by genetic studies.
Pittsburgh Compound B PET Imaging as a Pathophysiologic Marker of Brain Amyloid Deposition
Amyloid imaging with PET represents a major advance in AD diagnosis, by enabling the detection and quantification of pathologic protein aggregations in the brain. Pittsburgh compound B labeled with carbon 11 ( 11 C-PiB), an analogue of the amyloid-binding thioflavin-T, is the most extensively studied and best validated tracer. 11 C-PiB binds specifically to fibrillar β-amyloid (Aβ) deposits, amyloid plaques, and vascular amyloid, but not appreciably to other protein aggregates such as NFTs or Lewy bodies. 11 C-PiB binds nonspecifically to white matter, likely because of delayed clearance of the lipophilic compound from white matter. Using clinical diagnosis as the gold standard, the sensitivity of 11 C-PiB for AD diagnosis has been reported as 80% to 100%, with most studies reporting sensitivities of 90% or greater.
In most patients, the distribution of tracer uptake is diffuse and symmetric. The highest tracer uptake is consistently found in the prefrontal cortex, precuneus, and posterior cingulate cortex, closely followed by lateral parietal and temporal cortex and striatum, with lower tracer uptake in occipital cortex, globus pallidus, and thalamus. 11 C-PiB-PET can identify patients with MCI who have amyloid deposition and who may be considered to be at an early clinical phase of AD. Longitudinal studies showed that patients with MCI with significant 11 C-PiB retention are at higher risk of developing AD dementia, in contrast with patients with MCI without significant 11 C-PiB retention. 11 C-PiB-PET may be useful to distinguish cognitive deficit caused by AD from non-AD cognitive deficit.
PET imaging with 11 C-PiB may be useful in identifying atypical forms of AD, presenting either as a logopenic primary progressive aphasia or a posterior cortical atrophy. 11 C-PiB can also detect amyloid deposition in other dementia syndromes associated with β-amyloidosis to varying degrees, including cerebral amyloid angiopathy, or Lewy body dementia (LBD). In addition, amyloid PET imaging can improve the differential diagnosis of AD from FTD. The significance of a negative 11 C-PiB scan in a patient clinically diagnosed with AD is not yet clear, but it may be explained by 11 C-PiB binding that it is insufficient for in vivo detection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree