Fig. 4.1
A 3D isotropic fsPD SPCAE sequence of a 26-year-old soccer player with symptomatic FAI is provided with its multi-planar reconstruction in different planes (a–c). If in a clinical study only a few biochemical slides can be obtained, e.g., in a patient with CAM impingement, the interesting anterior-lateral orientation can be reconstructed in the isotropic sequences, and the thicker (~ 3 mm) biochemical slides can be obtained
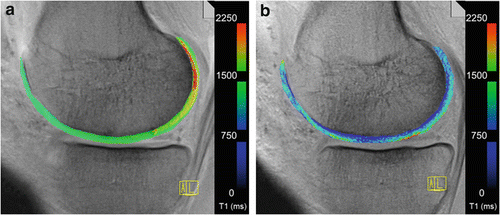
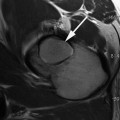
Fig. 4.2
A gradient echo T1 mapping sequence before (a) and after (b) i.v. Gadolinium (dGEMRIC) of a 36-year-old patient with suspicious early OA after repetitive smaller injuries of the lower extremity and chronic pain during running
Concluding preoperative MRI (respectively, optimal cartilage diagnosis) should contain of a set of cartilage-sensitive MR sequences, and if possible, a 3D-isotropic MR sequence and as well as (if possible) of biochemical MR sequences. Moreover, the rest of the joint has to be diagnosed in comparably high quality.
Clinical Applications to Visualize the Glycosaminoglycan Content
dGEMRIC displays the distribution of glycosaminoglycans (GAGs), one of the major macromolecular constituents of cartilage which appears to be a key factor for its mechanical function. GAGs are lost at early stages of OA and would need to be replenished with therapy [32]. To quantify GAGs, dGEMRIC utilizes the anionic contrast agent gadopentetate dimeglumine (Magnevist; Schering AG, Berlin, Germany). Due to abundant carboxyl and sulfate groups, GAGs are negatively charged too. Therefore, lost GAGs will be replaced by Gd(DTPA)2−, if given time to penetrate cartilage tissue. The distribution of Gd(DTPA)2− in tissues can be determined through MR measurements of the longitudinal relaxation time T1 for which an approximately linear inverse relationship with the GAG concentration has been demonstrated [16].
Recent clinical applications highlight the ability of dGEMRIC to demonstrate cartilage changes in patients with early OA [33], the influence of traumatic knee injuries, such as anterior cruciate ligament injuries, on the GAG content of cartilage [34], and differences in the tissue matrix of native cartilage and cartilage implants during follow-up of therapy [13, 35]. These studies show the applicability of dGEMRIC in a clinical background with different benefits in clinical studies and patient care:
The GAG content can be quantified and in larger patient cohorts it is possible (when a comparable protocol is used at a comparable magnet and coil) to get a noninvasive information of the quality of the cartilage layers.
Early grades of OA can be diagnosed and quantified which provides another measure in decision making, e.g., when planning a joint preserving surgery. Using this possibility, predictive values are available before treating the patient.
Therapy monitoring after various surgical or nonsurgical treatments is possible on a histological level without the need of a biopsy.
After cartilage repair, the maturation of the repair tissue can be assessed over time, and possible biomechanical statements (e.g., when the patient can return to high impact sports) can be answered.
After cartilage repair the quality of the repair tissue can be assessed noninvasively which provides a predictive value on the durability of the cartilage transplant and helps to assess the probability of failures [18, 36].
New therapeutical approaches can be assessed in their ability to treat cartilage pathologies noninvasively which is important for nonsurgical and surgical options. Especially upcoming treatment options might be assessed easier and in more detail (on a histological level).
These options are based on initial clinical studies in smaller patient groups [14, 37–43] and have to be verified in larger patient cohorts. Although these clinical applications are all preliminary, the strength of assessing the GAG content noninvasively by means of MRI and not by means of histological biopsies is not questionable. However, it has to be stated clearly that comparable analysis might be able in future approaches with other biochemical MR sequences possibly without the need for administering a gadolinium-based contrast agent and possiblenephrotoxicity.
In an investigation of the dGEMRIC technique in a clinical setup, a high-resolution, fast dual angle T1 mapping technique for 3D isotropic imaging was introduced to assess dGEMRIC index of the hip at 1.5 T, which provides sufficient resolution for accurate 2D reformatting [38, 44]. Previous studies on dGEMRIC have primarily used T1 maps of the cartilage from single-slice two-dimensional (2D) inversion recovery fast spin-echo (IR-FSE) sequences. This method has been validated in vitro and in vivo [45]; however, in a clinical setup and for the in vivo clinical application several shortcomings of the single slices acquisition sequence such as the lack of joint coverage, long acquisition times, and the need for post-processing have limited its clinical use and its accuracy for follow-up of localized lesions. Therefore, the 3D sequence for T1 and the dGEMRIC index was introduced for the hip [38, 44]. In an additional study the reproducibility of dGEMRIC was evaluated at the hip [46]. Two independent dGEMRIC examinations using the above high-resolution, fast dual angle T1 mapping were performed in ten asymptomatic adult volunteers with a very high interclass correlation coefficient (ICC) which proves the clinical applicability as well as the reproducibility in longitudinal patient measurements. Based on these results, a specific hip protocol could be introduced by the group around Kim and Mamisch and the cartilage degradation FAI patients could be quantified in a patient cohort [47]. In this study, OA grading according to Tönnis based on standard radiographs and morphological MRI was compared with quantitative dGEMRIC measurements in ten asymptomatic young adults and 26 FAI patients. Compared to asymptomatic volunteers, cam- and pincer-type FAI patients had significantly lower T1 values in the group without radiographic evidence of OA proving that a biochemical MRI technique like dGEMRIC is able to assess a subtle decrease in the GAG content of cartilage before morphological changes begin. In an earlier study, the research group of Kim et al. applied dGEMRIC technique in a large patient cohort suffering from hip dysplasia prior to hip preserving surgery by PAO and correlated it with clinically relevant parameters like pain (assessed with WOMAC questionnaire) and severity of dysplasia [10]. In a subsequent study, the same research group performed a cohort study on patients undergoing PAO for the treatment of hip dysplasia with the goal to identify radiographic, clinical, and magnet resonance imaging findings that best predict treatment failure [19]. Multivariate analysis identified the dGEMRIC index as the most important predictor of failure of the osteotomy. For each 100-ms increase in the dGEMRIC value, the likelihood of failure decreased by 77 %, indicating a dramatically improved chance of success in the early postoperative period if the patient had a higher dGEMRIC value prior to the surgery.
However as mentioned above due to the significant limitation of using contrast agent for dGEMRIC including very sever side effects like nephrogenic systemic fibrosis (NSF), it has a limitation in its use in large scale clinical trials or for screening of OA. Therefore, none-contrast techniques would be preferable in long term.
Here 23(Na)-sodium imaging allows measurement of the FCD and hence quantification of the GAG content which offers a method for noninvasive in vivo evaluation of joint cartilage similar to the dGEMRIC technique [48, 49]. However, sodium MRI is up to now based on its low signal (in contrast to standard MRI) dependent on ultra-high fields if an in vivo evaluation of joint cartilage has to be performed. Initial studies on its use in the knee and the ankle are available, whereas in the hip joint, based on the thin cartilage layers and the lack of availability of dedicated multi-channel coils, clinical sodium imaging protocols are not yet available. Another possible non-contrast measure of the GAG content in cartilage is chemical exchange saturation transfer (CEST). CEST is a versatile contrast enhancement mechanism for MR imaging, where the CEST effect depends on the molecular group that provides the exchanging proton(s). One application of the CEST contrast is biochemical cartilage imaging, where the CEST contrast is generated by protons of glycosaminoglycans (gagCEST) [50]. However, also CEST imaging has still its drawbacks in clinical routine mainly because of the relatively long scan times necessary to obtain a reliable CEST contrast in multi-slice examinations. Based on new CEST imaging methods, providing consistent contrast in 3D scans within clinically acceptable measurement times, its clinical applicability nevertheless has been shown in initial studies [48, 51]. The high correlation between the introduced gagCEST method and [23] Na imaging implies that gagCEST is a potentially useful biomarker for glycosaminoglycans. Initial, ongoing studies will prove its applicability not only in the knee joint, but also in the GAG quantification of hip joint cartilage.
Another biochemical MR marker, relaxation time in the rotating frame (T1 rho), has been reported to be a sensitive marker of the loss of proteoglycans in articular cartilage [52–54]. T1rho is a time constant that characterizes the magnetic relaxation of spins under the influence of a radiofrequency field that is parallel to the spin magnetization. Changes in T1 rho were observed in cartilage plugs that were chemically or enzymatically depleted of GAG, but not in collagenase-treated tissue [54]. On the other hand, Menezes et al. found no correlation between the cartilage T1ρ and GAG concentration [55]. Hence, T1rho has to be shown a valuable parameter in biochemical MR imaging of joint cartilage in the last years [53, 56]; its role as a replacement of dGEMRIC, however, is questionable. In a recent study, the clinical application of T1rho in the hip joint in patients with FAI has been shown and [57] acetabular hyaline cartilage changes in patients with FAI could be shown.
Concluding in the clinical applications to visualize the glycosaminoglycan content, dGEMRIC is still the standard, however, with the given disadvantages. Other sequences will show their potential in the quantification of GAG in knee and hip cartilage.
Clinical Applications to Visualize the Collagen Content and Orientation
Perhaps the most frequently implemented biochemical MR technique is the transverse relaxation time (T2) of cartilage as a sensitive parameter for the evaluation of changes in water and collagen content and tissue anisotropy [17]. Cartilage T2 reflects the interaction of water and the extracellular matrix on a molecular level. The collagen fiber orientation defines the layers of articular cartilage. Thus, the three-dimensional organization and curvature of the collagen network, influenced by water mobility, the proteoglycan orientation, and the resulting magic angle at 55° (with respect to the main magnetic field (B0)), influence the appearance of T226,71. Many studies especially in the knee joint, however also in the hip joint, show different clinical applications of T2 mapping and hence collagen-sensitive biochemical MRI [15, 22, 23, 58–61]. These studies show the applicability of T2 mapping in a clinical background with different benefits in clinical studies and patient care:
The collagen orientation and (in parts) the collagen content can be quantified and in larger patient cohorts it is possible (when a comparable protocol is used at a comparable magnet and coil) to get a noninvasive information of the quality of the cartilage layers.
Early grades of OA can be diagnosed and quantified which provides another measure in decision making, e.g., when planning a joint preserving surgery. However in comparison to dGEMRIC, for T2 mapping there is no uni-directional when degeneration is taking place. This makes it harder to really interpret slightly increased or decreased T2 values.
Therapy monitoring after various surgical or nonsurgical treatments is possible on a histological level without the need of a biopsy.
Especially for T2 mapping and all other collagen-sensitive MR techniques, a zonal evaluation of the cartilage is essential to provide a precise information if a hyaline-like cartilage structure is present.
Biomechanical MRI is relatively easily possible in clinical patient care and loading- or unloading can be visualized.
After cartilage repair, the maturation of the repair tissue can be assessed over time, and possible biomechanical statements (e.g., when the patient can return to high impact sports) can be answered.
After cartilage repair the quality of the repair tissue can be assessed noninvasively which provides a predictive value on the durability of the cartilage transplant and helps to assess the probability of failures [18, 36].
New therapeutical approaches can be assessed in their ability to treat cartilage pathologies noninvasively which is important for nonsurgical and surgical options. Especially upcoming treatment options might be assessed easier and in more detail (on a histological level).
Hence, collagen-sensitive biochemical MR techniques provide roughly comparable possibilities than GAG-sensitive techniques. A drawback of, e.g., T2 mapping, however, is the problem in the interpretation of, e.g., slightly decreased or increased values [62]; a clear benefit is the relatively easy clinical use and the relatively robust sequence without the need of contrast agent. A further advantage is the various biomechanical applications which are already available for clinical applications. For example, loading or unloading of cartilage areas can be assessed noninvasively and in vivo, where initial studies show a clearly different biomechanical response of healthy and altered (OA or after cartilage repair) articular cartilage to unloading [21, 63]. In biochemical or biomechanical collagen-sensitive MRI, the zonal evaluation of cartilage plays an important role. In healthy articular cartilage, an increase in T2 values from deep to superficial cartilage layers can be observed. Histologically validated animal studies have shown this zonal increase in T2 values as a marker of hyaline or hyaline-like cartilage structure after cartilage repair procedures within the knee73,74. To visualize this zonal variation in vivo, high spatial resolution is essential. In combination with a dedicated (multi-channel) coil nevertheless, T2 mapping in clinically applicable scan time could be achieved on most available MR magnets. In the hip joint, however, the resolution of the cartilage has to be mainly high enough to really delineate the femoral and the acetabular cartilage. Within e.g., the femoral cartilage it nevertheless remains challenging to assess a deep and a superficial cartilage layer.
In cartilage repair tissue T2 values have shown an increase in the early postoperative follow-up, which enables for visualization of cartilage repair tissue maturation20. Furthermore it has been shown that a zonal T2 evaluation is able to differentiate cartilage repair tissue after MFX and MACT [15]. Whereas cartilage repair tissue after MFX–histologically seen as fibrocartilage–shows no clear zonal increase from deep to superficial cartilage aspects, repair tissue after MACT—histologically reported as hyaline-like–shows a significant stratification.
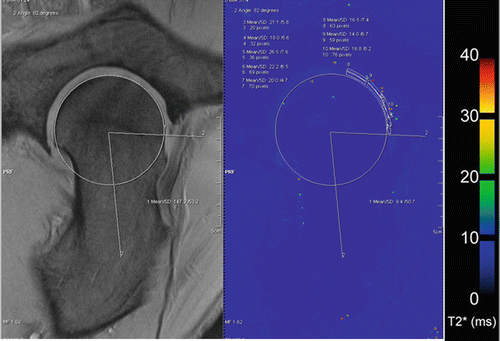
In addition to standard 2D multi-echo spin-echo T2 relaxation, T2*- weighted 3D gradient-echo articular cartilage imaging has shown reliable results in the evaluation of chondromalacia of the knee [64]. In recent studies, T2* mapping, with its potentially short scan times, was correlated to standard T2, and showed information comparable to that obtained for articular cartilage in the knee, but with overall lower T2* values (ms) [65, 66]. Furthermore, also for T2*, a clear zonal variation between deep and superficial cartilage layers was described for healthy cartilage; after cartilage repair using MFX, however, this stratification could not be found [65]. Thus, for standard T2, as well as for comparable techniques, zonal assessment of healthy and altered articular cartilage is crucial in the thick cartilage layers of the knee joint. In the hip joint compared to standard T2 mapping, T2* mapping has several benefits with its potential higher signal and its ability for 3D imaging. In a recent study by Bittersohl and coworkers, T2* values could be correlated in various histological severities of osteoarthritis [22]. However, and this has to be seen contrary to standard multi-echo spin-echo T2 (Fig. 4.3), T2* values decreased significantly with increasing cartilage degeneration (Fig. 4.4) [22]. This study is nevertheless a good example that all results and conclusions of biochemical MRI have to be seen with caution and that more histological “gold-standard” studies are needed in future. Hence, when applying biochemical MRI to clinical studies or to patient care, the physicians have to learn to interpret the results. Nonetheless these results will for sure provide new insights into the pathophysiological pathway of, e.g., cartilage degeneration.
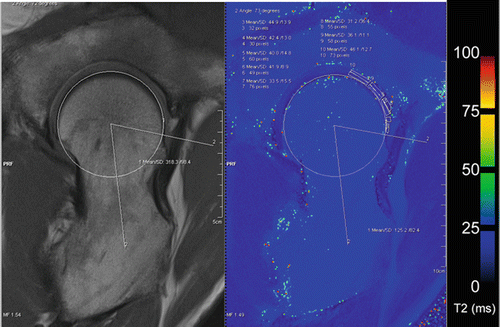

Fig. 4.3
A multi-echo spin-echo T2 mapping sequence of a 33-year-old soccer player with symptomatic FAI. The images out of a clinical cohort were planned on an isotropic sequence (see above), and by using quantitative T2 values, the cartilage quality was scored