Fig. 10.1
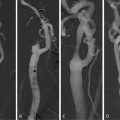
Measuring the degree of luminal narrowing using the NASCET method involves measuring the minimal residual lumen diameter at the level of maximal stenosis (double arrow), measuring the lumen diameter at the level of the distal cervical internal carotid artery, and reporting the difference of the two as a percentage of the later
An alternative method to calculating directly percent of stenosis has been proposed by Fox et al. [33] (Table 10.1). It consists of measuring the minimal luminal diameter in millimeters and infer the corresponding degree of stenosis. A minimal lumen of 1.3 mm corresponds with 70% stenosis and a minimal lumen of 2.2 mm with 50% stenosis [34].
Table 10.1
Correspondence between minimal luminal diameter in millimeters and degree of stenosis in %, as proposed by Fox et al. [33]
Stenosis (mm) | Stenosis (%) |
|---|---|
2.2 | 50–55 |
2.0 | 54–59 |
1.7 | 61–66 |
1.5 | 66–70 |
1.3 | 70–74 |
1.0 | 77–80 |
0.9 | 80–82 |
0.6 | 86–88 |
0.4 | 91–92 |
0.2 | 95–96 |
Recently, software has become available on post-processing workstations that performs semiautomatic segmentation of the carotid artery lumen on CTA studies and automatic quantitative measurements of diameter and area. This approach could potentially alleviate some of the limitations listed above, and the use of such software has recently been validated [35].
4 Carotid Wall Characterization Using CT
The widespread use of the luminal narrowing to assess the severity of carotid artery stenosis is based primarily on the results of several randomized clinical trials that demonstrated a reduction in the risk of ischemic stroke in patients with luminal stenosis of ≥70% (assessed on conventional angiograms), after carotid endarterectomy compared with medical treatment alone [13, 15, 22]. However, ≥70%-carotid stenosis occurs in fewer than 10% of patients, whereas <70%-carotid stenosis is extremely frequent in the general population (70% in men and 60% in women over 64 years of age) [1, 2]. In patients with <70% carotid stenosis, high-resolution lumenography fails to provide any insight into the associated risk of stroke, because angiography is able to detect atherosclerosis only when >40% of the area of the vessel wall is occupied by the plaque [36].
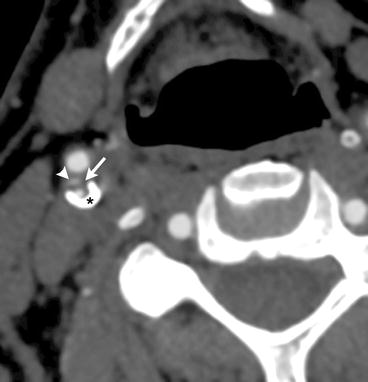

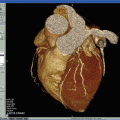
Fig. 10.2
CTA image showing a complex plaque at the right internal carotid artery bulb, with an ulceration (arrowhead), lipid-rich necrotic plaque (arrow), and calcified plaque (star)
Luminal narrowing on conventional angiogram is only an indirect measure of the carotid atherosclerosis process as it occurs in the vessel wall, not the lumen. Angiography is not able to detect atherosclerosis at early stages because luminal narrowing begins only when >40% of the vessel wall is occupied by plaque [36, 37]. However, plaque complications that result in strokes can occur in these early stages of atherosclerosis, a phenomenon that has also been described for coronary arteries (where a significant proportion of acute myocardial infarcts occur as a result of plaque rupture in coronary arteries with normal or subcritical narrowing) [38, 39]. For this reason, parameters other than luminal narrowing are needed to predict the risk of stroke more reliably, particularly in patients with <70% stenosis. Plaque morphology and composition have been suggested as a complement to luminal narrowing measurements for assessing carotid atherosclerotic disease, giving rise to the concept of “vulnerable plaque” [40–44]. A number of carotid plaque morphological features have been suggested as potential markers of the “vulnerable plaque” and are possibly associated with an increased risk of stroke, the most studied of which being the common carotid artery (CCA) intima–media thickness [1, 2, 45–49]. Also, embolic phenomena have been reported as being associated with thinning and subsequent ulceration of the fibrous cap on the surface of the atherosclerotic plaque [40, 44, 50–52] resulting in release into the parent vessel of necrotic lipoid debris from the plaque substance, especially in the case of a high plaque lipid content [53, 54]. On the contrary, carotid plaque calcifications appear to be protective [55, 56].
Noninvasive in vivo imaging of carotid atherosclerotic plaques holds considerable promise for clinical decision-making and treatment [53, 57, 58]. Such imaging has classically been achieved using ultrasound [1, 2, 45–49] and also magnetic resonance imaging (MRI) [13, 59–64].
More recently, the ability of modern, multidetector-row, isotropic resolution CTA (Fig. 10.2) studies to assess the histological composition (including non-calcified components) and characteristics of carotid artery atherosclerotic plaques were demonstrated, using histology as the gold standard. In this study, there was 72.6% agreement between CTA and histology for carotid plaque classification, perfect concordance for calcifications, and good correlation with histology for large lipid cores. CTA was also accurate in the detection of ulcerations and in the measurement of fibrous cap thickness [51]. Utilizing the standardized, computerized assessment of CTA studies described above, Wintermark et al. performed a retrospective, cross-sectional study to identify the CT features of carotid atherosclerotic plaques that were significantly associated with the occurrence of ischemic stroke [65]. This study revealed that a small number of carotid wall CT features were significantly associated with acute carotid stroke. Specifically, increased risk of acute carotid stroke was associated with an increased wall volume, a thinner fibrous cap, a higher number of lipid clusters, and lipid clusters closer to the lumen [65]. The number of calcium clusters was a protective factor [65]. Unfortunately, there were several limitations to the pilot study. The design of the study was cross-sectional, and it involved only a small number of patients. The authors concluded that their results needed to be confirmed in a large cohort study.
In a subsequent study, involving a retrospective cohort of a large sample of patients, 14 patients who developed a new, carotid ischemic stroke between the time when they underwent their baseline CTA of the neck and a follow-up imaging study of the brain were identified. Three risk factors that would predict 10 of the 14 carotid ischemic strokes were identified, including two clinical risk factors (age ≥ 75 and antihypertensive treatment) and one CT feature of carotid atherosclerotic disease—the maximal carotid wall thickness > 4 mm. This new rule appears straightforward to remember and apply clinically.
5 Conclusion
A better understanding of the role of carotid plaque components in predicting ischemic stroke will be important in designing future treatment trials for carotid artery stenosis. A decade ago, studies demonstrated a clear advantage in certain populations of endarterectomy compared with medical therapy. However, these studies were conducted before the widespread use of medical treatments that have been demonstrated to reduce stroke risk in patients with vascular disease, such as clopidogrel; extended-release dipyridamole; and aspirin, statins, and more aggressive blood pressure control [66].
With the development of both improved medical therapy and less invasive percutaneous stenting approaches over the last few years, some of the same questions must be addressed again [1]. Should our hypotheses prove correct, these results will provide a relevant, standardized, and validated method of interpreting the carotid artery wall findings using a routine imaging technique that could be used in future trials to monitor the carotid wall composition, both to select patients for treatment and to determine whether drug treatments are effective in altering the size and composition of the atheroma.
References
1.
2.
3.
4.
5.
6.
González RG, Hirsch JA, Koroshetz WJ, Lev M, Schaefer PW, SpringerLink (Online service) (2006) Acute ischemic stroke imaging and intervention. Springer, BerlinCrossRef
7.
8.
9.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree