, Gila Perk2, Natesa G. Pandian3, Hans-Joachim Nesser4 and Itzhak Kronzon2
(1)
Department of Cardiology, Cardiocentro Ticino, Lugano, Switzerland
(2)
Non-Invasive Cardiology, Lenox Hill Hospital, New York, NY, USA
(3)
Tufts University School of Medicine, Boston, MA, USA
(4)
Department of Medicine Elisabethinen, Teaching Hospital, Linz, Austria
Abstract
Ventricular septal defect (VSD) is a rare but deadly mechanical complication of acute myocardial infarction (AMI). Although significantly lower than with conservative management, in-hospital mortality after surgery remains as high as 47%, and dehiscence of the patch requiring a second operation is not infrequent because the sutures may teat out of the friable support around the defect. In today’s era of percutaneous interventions, percutaneous closure is considered a valid alternative to surgical repair in patients with small to medium VSDs, since the occlusion device may provide a definitive treatment. Two-dimensional TTE/TEE color Doppler provides the most rapid diagnosis by demonstration of a discontinuity in the ventricular septum with left-to-right shunt. 3D TEE appears to be a very valuable tool in showing the defect as it anatomically is: a hole through the septum. In this chapter we describe 3D TEE anatomy of ventricular septum, 3D TEE imaging of post-AMI VSDs, and the current use of 3D TEE during percutaneous closure with catheter-based devices.
Electronic supplementary material
The online version of this chapter (doi:10.1007/978-1-4471-4745-9_7) contains supplementary material, which is available to authorized users.
Ventricular septal defect (VSD) is a rare but deadly mechanical complication of acute myocardial infarction (AMI). Before thrombolysis, post-AMI VSD occurred in as many as 1–3 % of patients. With the advent of early reperfusion strategies, the incidence of this complication has dropped significantly to less than 0.2 %. Septal rupture tends to occur 3–8 days after the AMI, but it may occur within 24 h or as late as 2 weeks afterward. Three mechanisms are reported, which, of course, may merge in individual patients: septal rupture occurring in the first 24 h is usually due to a large intramural hematoma that dissects into the tissue, leading to an abrupt laceration. Later ruptures may result from erosion of the infarcted septal wall (with subsequent tear) by lytic enzymes produced by neutrophils in the infarcted area. A third mechanism involves the formation of a thin septal aneurysm, with perforation occurring in its central portion.
In most patients, the defect consists of a discrete hole with a direct, through-and-through communication across the septum. Complex defects are characterized by serpiginous, convoluted tracts within the necrotic tissue. Less commonly, the post-AMI VSD may be associated with a dissection of the interventricular septum or right ventricular free wall. Septal rupture in a patient with anterior AMI tends to be apical, with a discrete simple hole. In patients with inferoposterior AMI, the defect involves the basal inferoposterior septum and often has a more complex geometry. The size of the defect may vary from few millimeters to several centimeters and is the primary cause of the magnitude of the left-to-right shunt. Large defects lead to the development of right ventricular dysfunction, pulmonary hypertension, congestive heart failure, low cardiac output, and fatal cardiogenic shock. Some decades ago, there was a tendency to treat patients with post-AMI VSD nonsurgically in the acute phase. A waiting period of 3–6 weeks was supposed to be necessary to allow the necrotic and friable margins of the defect to be replaced by robust connective tissue that would be more receptive to sutures. The mortality rate with this strategy was as high as 50 % at 1 week and up to 94 % at 1 month. Even apparently stable patients with small defects, when treated conservatively, may experience an unpredictable deterioration (due to extension of the defect, abrupt right ventricular failure, or both) leading to cardiogenic shock and death. Thus, current American and European guidelines suggest immediate operative intervention regardless of the patient’s clinical status. In the past two decades, developments in myocardial protection, improved prosthetic materials, and refined surgical techniques (i.e., infarctectomy, excision of all necrotic and friable margins, and reconstruction of the ventricular septum with Dacron patches) have certainly contributed to an improved surgical outcome (Fig. 7.1).
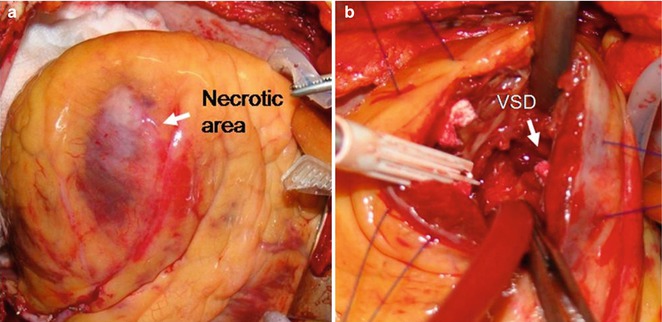

Fig. 7.1
(a) The exposed heart and apical infarction. (b) The ventricular septal defect (VSD) is shown through the apical incision (Modified with permission from Faletra FF, de Castro S, Pandian NG, et al. Atlas of real time 3D transesophageal echocardiography. London: Springer-Verlag London Limited; 2010. p. 153)
Inotropic support and, when necessary, the use of intra-aortic balloon counterpulsation may stabilize the patient during transportation or preparation for surgery. Coronary artery bypass grafting is recommended for patients with multivessel coronary artery disease. Although in-hospital mortality is significantly lower than with conservative management, it remains as high as 47 % after surgery (see GUSTO trial), and dehiscence of the patch requiring a second operation is not infrequent because the sutures may tear out of the friable support around the defect.
In today’s era of percutaneous interventions, some patients may benefit from catheter-based percutaneous closure of the septal rupture, but the role of percutaneous intervention in these patients is yet to be clarified. Unlike closure of congenital VSD, percutaneous closure of post-AMI VSD has several drawbacks. Although the procedure does not require the direct manipulation of the necrotic tissue surrounding the defect, any attempt to pass the stiff closure device through the defect may tear its fragile borders and thus increase the size of the hole. In patients with very basal defects, positioning and expanding the device may impinge on tricuspid or mitral valve leaflets, causing an unacceptable valve malfunction. Finally, in patients with inferior/posterior AMI, the septal rupture is usually near the base of the right and left ventricular free wall, which may be at least partially involved in the necrotic process; when the device is expanded, it may place excessive tension on the free wall, with risk of further tearing and defect expansion, or free wall distortion and rupture.
Currently, percutaneous closure is considered a valid alternative to surgical repair in patients with small to medium VSDs, in whom the occlusion device may provide a definitive treatment. Conversely, patients with very large defects should undergo immediate operation because of a high risk of device embolization. The procedure may also be accomplished in patients with residual leak after surgical closure, as bridge to a safer surgical correction, or in patients with unacceptable surgical risk because of age, comorbidity, or end-organ dysfunction.
Two-dimensional transthoracic/transesophageal echocardiographic (TTE/TEE) color Doppler provides the most rapid diagnosis by demonstrating a discontinuity in the ventricular septum with left-to-right shunt. Color Doppler may discover the abnormal shunt from the left ventricle to the right ventricle even when the anatomic grey-scale image does not show any distinct interruption in the septal contour. The scanning plane may in fact not correspond to the site of the defect, which can be revealed only by nonconventional, off-axis planes. Furthermore, the size and the shape of the defect are difficult to appreciate with a tomographic technique. In this respect, three-dimensional (3D) TEE and 3D color Doppler appear to be very valuable tools in showing the defect as it anatomically is—a hole through the septum.
This chapter reviews the 3D TEE anatomy of the ventricular septum, 3D TEE imaging of post-AMI VSDs, and the current use of 3D TEE during percutaneous closure with catheter-based devices.
7.1 Three-Dimensional TEE Imaging of Normal Ventricular Septum
In the normal heart, the ventricular septum has a fibrous component (the membranous septum) and three muscular components: the inlet septum, the outlet septum, and the trabecular septum. These designations are arbitrary, as there are no clear anatomic demarcations. Normally, the ventricular septum is curved, with its convex surface towards the right ventricle. Consequently, the inlet portion and the outlet portion are on different planes to one another (Fig. 7.2a). Compared with other portions of the septum, the trabecular portion is characterized by irregularly arranged trabeculations. The membranous septum is located in the interleaflet triangle immediately below the right and noncoronary aortic sinuses (see Fig. 5.17). Of note, the upper part of the right outlet septum is actually the subpulmonic muscular infundibulum and not a septal structure.
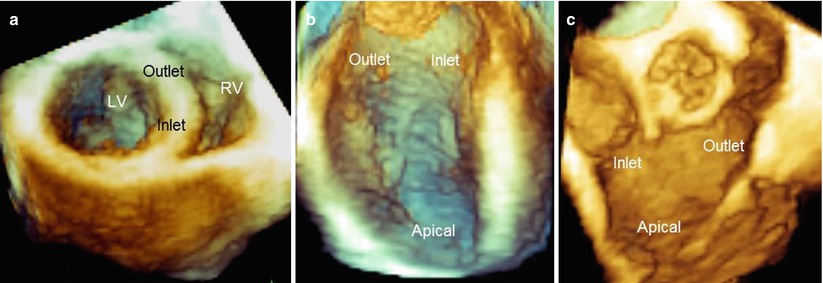

Fig. 7.2
(a) Three-dimensional TEE image of the septum in a perspective similar to short-axis view, showing the curved arrangement of the septum, so that the inlet and outlet segments are situated on different planes. (b, c) Left and right sides of the ventricular septal surface visualized from the en face perspective. LV left ventricle, RV right ventricle
Three-dimensional TTE/TEE can visualize cardiac surfaces such as the atrial septum (see Chap. 2) and ventricular septum in a unique perspective: the en face view. This perspective is unavailable when using tomographic planes such as those provided by conventional two-dimensional (2D) TTE/TEE. Thus, the left and right sides of the different components of the septum can easily be visualized (Fig. 7.2b, c).
7.2 Three-Dimensional TEE Imaging of Post-AMI VSDs
The en face perspective greatly facilitates the detection of post-AMI VSDs. Figure 7.3 shows an apical VSD from a left and right perspective, with the rupture consisting of a single, ellipsoidal perforation located the same level on both sides of the septum (Videos 7.1 and 7.2).
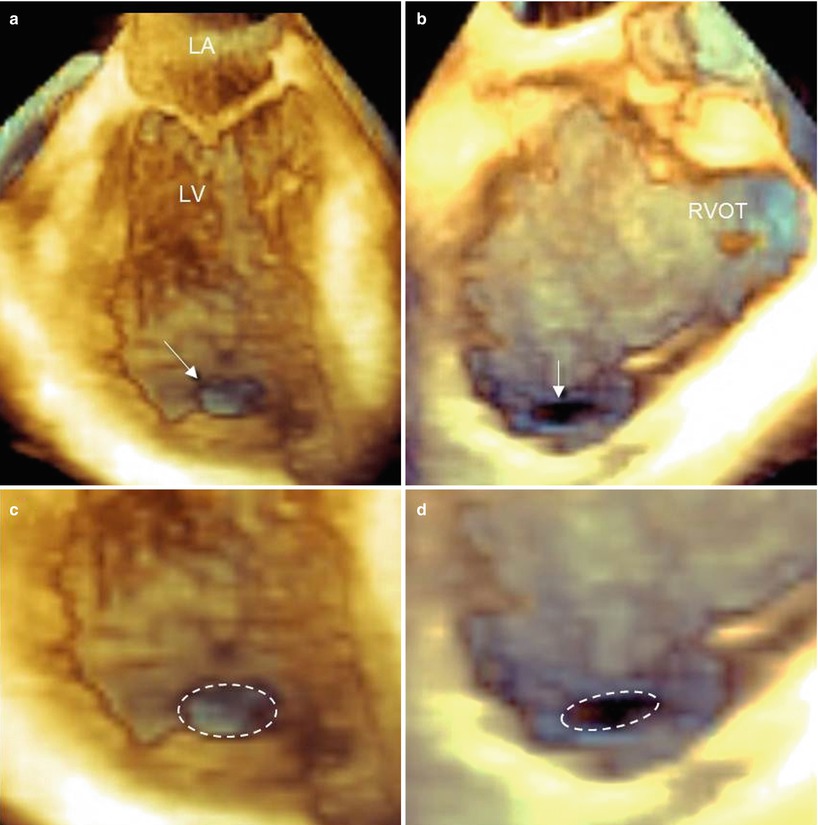

Fig. 7.3
Three-dimensional TEE images of an apical, ellipsoidal VSD visualized from the left (a) and right (b) ventricular perspective (arrow), and magnified images from the left (c) and right (d) perspective. The dotted line delimits the borders of the defect, emphasizing its elliptical shape. LA left atrium, LV left ventricle, RVOT right ventricular outflow tract
Of course, the en face view is not the only available perspective. The pyramidal data set acquired with 3D TEE is amenable to online and offline processing (cropping, rotations, angulations, etc.), allowing the visualization of defects from a countless number of angles. The richness of perspectives permits selection of the most favorable views. An easy and immediate perception of the site and size of the defect is of great importance in acute settings, when decisions regarding treatment should be taken in a short time. In this regard, the anatomic, specimen-like images provided by 3D TEE are much more quickly and easily interpreted by interventionists and surgeons than the conventional 2D planes (Fig. 7.4).
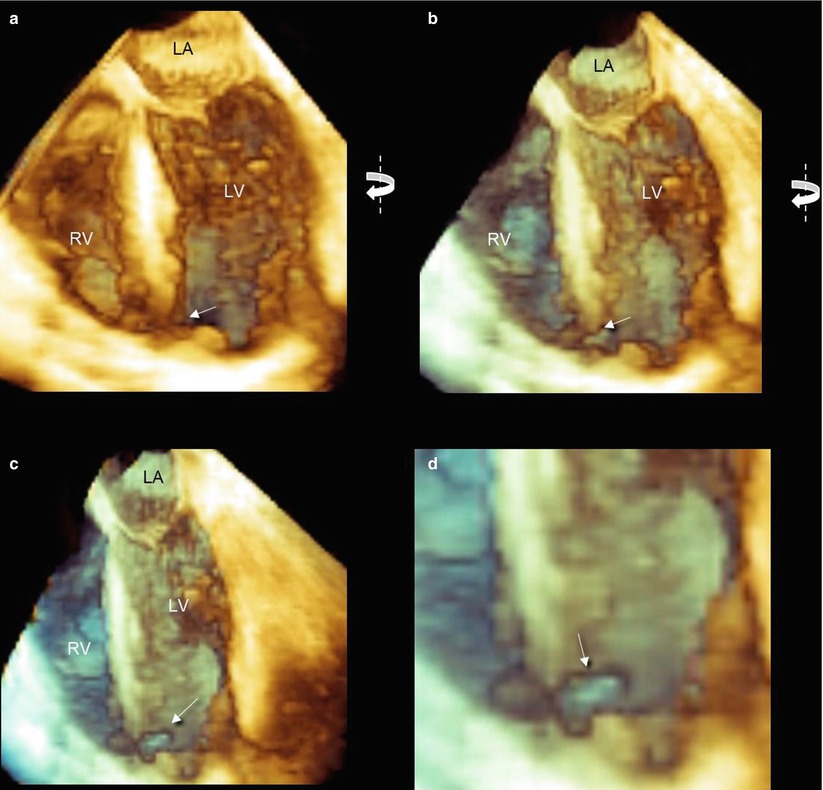

Fig. 7.4
Three-dimensional TEE images. (a) Classic four-chamber view. (b, c) Simple right-to-left rotation around the y axis may progressively reveal an apical ellipsoidal defect in en face view (arrow). (d) A magnified image from panel c shows the defect (arrow). LA left atrium, LV left ventricle, RV right ventricle
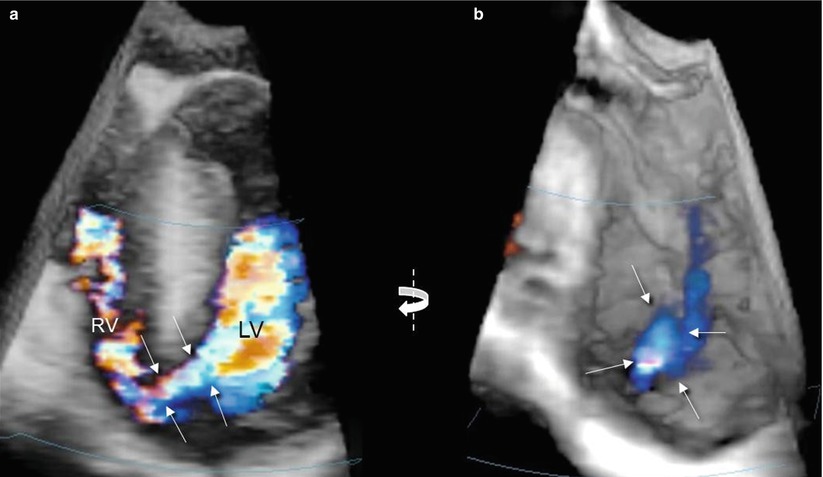
The use of 3D color Doppler may help in many ways. Different from 2D color Doppler, the addition of the third dimension allows the depiction of the entire course of the jet from the left to the right side of the septum (Fig. 7.5a). From the en face view, the borders of the defect may be precisely delineated by the color Doppler (Fig. 7.5b).
Get Clinical Tree app for offline access