COCHLEAR AND AUDITORY BRAIN STEM IMPLANTS
KEY POINTS
- Cochlear implant planning is a complex process of which imaging is only one component.
- Computed tomography and magnetic resonance imaging can help assess the potential candidacy for implantation and significantly influence operative planning.
- Precise and comprehensive evaluation of specific imaging features is required for the preoperative assessment.
- Imaging can contribute to the evaluation of post implant failures and complications in a limited number of cases.
Hearing loss is a very common sensory impairment affecting all age groups. It is most common in the elderly. Hearing loss can be managed effectively in many cases with hearing aids. If that approach fails or is not an option, a cochlear implant may be considered to restore an adequate level of hearing.
The cochlear implant changes sound to an electrical signal. That signal is sent to electrodes that are wound around the modiolus of the cochlea to the cells of the spiral ganglion from where the signal is propagated more proximally.
Psychological, social, physiologic, and anatomic factors determine the success of cochlear implants; thus, a comprehensive preoperative evaluation is required. Technologic improvements constantly expand the indications for cochlear implantation. Implants were originally used to help profoundly deaf individuals but are now used to improve compromised hearing in an ever growing population. For instance, implants originally were restricted to those over 2 years of age, but currently that threshold has been lowered to 12 months of age. Auditory stimulation at an early age is desirable since this plays an important role in the development of language and speech.
Whereas earlier only deafness or profound hearing loss was treated with cochlear implantation, nowadays electric acoustic stimulation (EAS)—a method that combines electric stimulation of high-frequency portions of the cochlea with amplified acoustic stimulation of low-frequency hearing—is considered.1
Auditory brain stem implants (ABIs) bypass the inner ear and directly stimulate the auditory pathway at the level of the cochlear nucleus complex. It is typically used in neurofibromatosis type 2 (NF-2) patients, even before deafness has occurred. Indications for ABI are expanding, and its use has been advocated for treatment of profound hearing loss or deafness in totally ossified cochleae, posttraumatic deafness, severe congenital malformations of the inner ear, and in the case of bilateral cochlear nerve hypoplasia or aplasia.2,3
CAUSES OF HEARING LOSS
The many causes of sensorineural hearing loss have been discussed in Chapters 106, 107, 117, 118, and 133 through 135. These include etiologies as diverse as genetic syndromic and nonsyndromic conditions; infectious diseases, most commonly due to viral and bacterial meningitis; pharmaceutical ototoxicity; trauma; hyperbilirubinemia due to nerve or brain toxicity; auditory neuropathy associated with prematurity and its complications; Ménière disease associated with primary or secondary endolymphatic hydrops; noise; and presbycusis (loss due to aging). Hearing loss associated with all of these conditions may benefit from cochlear implantation.
EVALUATION
Pure-tone audiometry and speech audiometry are the mainstays of clinical testing. Criteria are derived from these tests to establish whether the patient is a physiologic candidate for an implant. Particularly in pediatric candidates, objective audiometry, especially auditory brain stem responses, plays a key role. The actual hearing threshold that qualifies for implant funding may vary with patient age and third-party payer guidelines. Other less specific criteria may also be used. For instance, a prelingual deaf child may become a candidate when auditory skills do not develop following amplification and a reasonable period of attempted rehabilitation.
Once candidacy is established by reasonably objective criteria and the social and psychological setting is deemed appropriate, imaging is used to provide gross anatomic information that may establish the cause of the hearing loss as well as to help determine whether the patient remains a candidate. If so, the imaging data may help plan the surgical approach.
IMAGING WITH PLAIN FILMS, MAGNETIC RESONANCE, AND COMPUTED TOMOGRAPHY
Pretreatment Evaluation
Pretreatment imaging with computed tomography (CT) and/or magnetic resonance (MR) allows the visualization of the entire auditory pathway. MR is more complete than CT as a single test in this regard, lacking only the bone detail of the latter. The CT or MR results may alter the choice of side of implantation or suggest the more appropriate electrode selection.
It is reasonable to ask whether CT or MR should be the primary or only imaging study done for precochlear implant evaluation. It is clear that CT and magnetic resonance imaging (MRI) render complementary information in the preoperative assessment of cochlear implant candidates. The issue is what is truly necessary for medical decision making. The surgeon needs to be informed about the patency of the cochlea and the presence of cochlear and vestibular anomalies or bone dysplasias as well as any retrocochlear disease. Anatomic variations, especially those of the facial nerve or along the usual mastoid and middle ear approach to the preferred round window insertion site, that might alter the surgical approach should be noted. CT provides information about the bony labyrinth, the vestibular aqueduct, and anatomic variants of the mastoid and vascular structures (Figs. 104.4, 104.5, 104.34, 104.59, and 136.1). MRI will identify cochlear and retrocochlear soft tissue abnormalities, such as cochlear fibrosis in labyrinthitis, otospongiotic foci in otosclerosis, absence or extreme atrophy of the cochlear nerve, and brain pathology along the auditory pathways. The latter includes pathology of developmental, vascular, demyelinating, neoplastic, and traumatic origin (Fig. 136.2). Superficial siderosis is a relatively rare condition that manifests in almost all patients with progressive sensorineural hearing loss and eventually leads to deafness. The condition is the result of chronic or repeated subarachnoid bleeding. Deposition of hemosiderin and iron leads to gliosis, neuronal loss, and demyelination affecting mainly the cerebellar cortex and cranial nerve VIII because of its long glial cisternal segment. The related symptoms are cerebellar ataxia, pyramidal tract signs, and dementia in a late stage of disease. Sporadically, anosmia is noted due to involvement of the olfactory nerve. Although several cases of successful cochlear implantation have been reported, not all patients benefit from this treatment4–6 and preoperative patient counseling is needed.


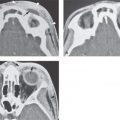
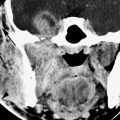
FIGURE 136.1. Preoperative assessment for cochlear implantation—anatomic variants that might influence the surgical approach. Most commonly, anatomic variants of the mastoid and venous vascular structures are encountered. A, B: A high-riding, dehiscent (arrow) jugular bulb may complicate the access to the round window. In this case, additionally a jugular diverticulum (*) is present. (A, axial computed tomography [CT], B, coronal CT). C, D: Anatomic variations of the sigmoid sinus will influence the surgical approach through the mastoid. In (C), there is lateral displacement of the sigmoid sinus; in (D), there is lateral displacement of the sigmoid sinus and diminished access anteriorly due to an arachnoid granulation (*). E: Anatomic variations in mastoid pneumatization may produce surgical challenges: A low-lying temporal lobe narrows the surgical field through the mastoid (arrow). A large hypotympanic mastoid cell (*) may mimic the round window niche during surgery, leading to an aberrant insertion.
The abnormalities detected with MRI related to cochlear patency and the auditory pathway are more likely to influence the selection of candidates and the preferred side of surgery than the findings on CT. Both MRI and CT should be considered for prelingual deafness or complex pathology. If a group prefers only CT, then MRI routinely should at least be added in all cases of previously documented meningitis in order to evaluate cochlear patency or in prelingual deafness to establish the presence of a normal cochlear nerve. These relative values and roles of CT are currently under study, and the safest course of action seems to be doing both studies.7–10 MRI has more risk and expense in children under about 8 years old because of the frequent necessity for the study to be done under deep sedation or anesthesia.
Posttreatment
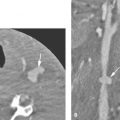
Plain films can provide an overview of the implant and be used to look at gross placement within the cochlea and integrity of the implant. An electrode array consists of a Silastic carrier with several contacts; the number and spacing of these contacts differ between manufacturers. This electrode array must be positioned in the cochlea with regular spacing of the contacts and without breakage of the carrier. Several techniques of skull base radiography have been used, such as the Stenver view or the transorbital view (Fig. 136.3A). The cochlear view was designed to optimize the visualization of a cochlear implant: By directing the x-ray beam to the cochlea and parallel to the modiolus (axis of the cochlea), an image was obtained within the plane of the implant. This is achieved by positioning the patient with the head resting on the forehead, nose, and zygomatic bone on the implanted side. The midsagittal plane should form an angle of 50 degrees with the plane of the film, while the horizontal plane contains the infraorbital-meatal lines11 (Fig. 136.3B). This technique also allows for objective documentation of the insertion depth of an implant. This is of particular interest for frequency mapping (correlating the perceived frequencies to the electrode position within the tonotopically organized cochlea) and optimizing speech-processing strategies.



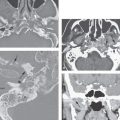
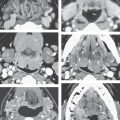
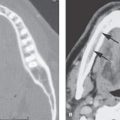
FIGURE 136.2. Preoperative assessment for cochlear implantation—retrocochlear diseases in three patients. A–D: Patient 1. In (A) and (B), the T2-weighted (T2W) image of the brain shows a widened Sylvian fissure on the left and cortical dysplasia/migrational abnormalities involving the auditory cortex. In (C) and (D), the three-dimensional steady state (SS) images show normal development of the inner ear, and the internal auditory canal is seen. There is, however, hypoplasia of the cochlear nerves, most likely secondary to the migration abnormality of the cortex. The patient was deaf. E, F: Patient 2. This patient complained of sensorineural hearing loss (SNHL) and epilepsy after hemorrhage involving the left auditory cortex (arrow in E). The underlying cause of the hemorrhage was a micro arteriovenous malformation; on digital subtraction angiography, early venous drainage to the transverse sinus is seen (arrows) (F). G, H: Patient 3 had progressive SNHL, and gait disturbances 12 rims of dark signal intensity are seen on the cerebellar folia and brain stem surface on T2W images (arrows). Cerebellar atrophy and areas of hyperintensity are seen within the parenchyma. The patient had a history of skull base fracture as a probable cause of superficial siderosis. Note the long cisternal segment of cranial nerve VIII. This patient showed good speech perception after implantation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree