COCHLEOVESTIBULAR NERVE: VASCULAR CONDITIONS
KEY POINTS
- Magnetic resonance imaging and computed tomography are commonly performed to look for abnormalities of the cochleovestibular nerve, and the results frequently drive medical decision making.
- Pathology in this region may be diverse, but the vast majority of cases are straightforward diagnostic situations.
- Neurovascular compression should be diagnosed cautiously with consideration of the presence of symptoms and imaging findings that are together suggestive of a vascular compression syndrome.
Slow-flow malformations, most commonly called hemangiomas, are uncommon lesions of the cochleovestibular nerve (CVN). These most commonly involve the facial nerve in the temporal bone but may secondarily involve the internal auditory canal (IAC) and CVN. Cerebellopontine angle (CPA) dural or pial–dural vascular malformations may involve the CVN and/or the facial nerve; these are rare lesions. Microvascular compression is believed to be a cause of CVN-related symptoms, including sensorineural hearing loss, tinnitus, and vertigo. Other vascular malformations such as an aneurysm rarely present with CVN symptoms.
ANATOMIC AND DEVELOPMENTAL CONSIDERATIONS
Applied Anatomy
A diligent search of the entire course of the CVN is essential for evaluating imaging studies performed in patients with symptoms referable to the nerve. Exclusion of causative pathology with a high degree of confidence is the usual goal of the study.
In order to accomplish such a search, the computed tomography (CT) and magnetic resonance imaging (MRI) anatomy of the CVN must be completely understood, including its brain stem nucleus, its course through the brain stem, its cisternal segment, its course through the IAC, and points of distribution of its three main branches to the cochlea and vestibular system. This anatomy is presented in detail in Figures 104.11 through 104.14 and Chapter 104.
IMAGING APPROACH
Techniques and Relevant Aspects
MRI and CT in this anatomic region almost always requires the highest possible spatial resolution. These factors used to develop optimal protocols are discussed in detail in Chapters 1 through 3. Specific protocols for magnetic resonance (MR) and CT studies for investigating CVN and CPA problems in general appear in Appendixes A and B. Any such MR study must include a three-dimensional (3D) steady state acquisition with nominal section thickness #1 mm that can be analyzed in any plane. The same type of CT data analysis is necessary.
The CVN and CPA are typically studied with dedicated contrast-enhanced studies of the temporal bone. These studies must also include definitive high-resolution images of the posterior fossa and CVN. MRI studies should also always include images that are as definitive as possible for evaluating the membranous labyrinth of the inner ear and related structures such as the vestibular aqueduct. The inner ear is the “end organ” of the CVN innervation, and its status will almost always be in question when potential pathology of the CVN and CPA is suspected. Non–contrast-enhanced computed tomography (NCCT) only may be used to evaluate the inner ear anatomically, but that is the subject of pathologic processes that are discussed in other chapters, even though they may be functionally related to CVN and CPA problems.
Computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) may be included when a vascular lesion or compressive effect by a blood vessel or aneurysm is known or suspected usually based on already existing imaging data, as it is fairly difficult to anticipate when such a study may be fruitful in advance.
Catheter angiography is necessary to produce definitive images of the angioarchitecture of arterialized lesions and assess flow dynamics, although 320 multidetector computed tomography (MDCT) is rapidly eliminating the need for catheter as a diagnostic tool in many of these cases.
Pros and Cons
MRI should be done first, as it most confidently excludes causative pathology at all CVN segments (i.e., the brain stem, cisternal, IAC, and inner ear segments) from its brain stem nucleus to the inner ear. MRI is more sensitive than CT for excluding intra-axial pathology such as demyelinating disease, pia-arachnoid diseases, and small neoplasms of the nerve that might involve the cisternal segment or the segment within the IAC as well as perineural spread of cancer. Steady state 3D sequences can screen very nicely and perhaps better than even advanced CTA for potential vascular compression nerve irritation.
CT may be done as a supplement to most confidently exclude other pathology that might mimic that of the CVN, such as superior semicircular canal dehiscence. This supplemental imaging may be done as a routine study when MRI findings are suspicious for an anatomic abnormality or related to bone, such as otosclerosis that creates a mixed hearing loss.
Even current MDCT with <320 MDCT capability cannot, depending on the size of the coverage area, produce definitive images of the angioarchitecture of arterialized lesions and cannot really assess flow dynamics as well as catheter angiography. Also, MRA cannot produce definitive images of the angioarchitecture of arterialized lesions and cannot definitively assess flow dynamics as well as catheter angiography.
Controversies
There is significant controversy with regard to neuropathy caused by vascular and microvascular compression. This is discussed in detail in Chapter 9.
SPECIFIC DISEASE/CONDITION
Hemangiomas and Other Vascular Malformations of the Cochleovestibular Nerve
Etiology, Prevalence, and Epidemiology
Vascular malformations, both high and low flow, affecting the CVN are all uncommon sporadic lesions (Figs. 135.1 and 135.2).
Hemangiomas account for a substantial percentage of the benign masses that will produce facial nerve weakness; however, they are an exceedingly rare cause of CVN dysfunction.1 High-flow vascular malformations are more common but still relatively unusual lesions in the spectrum of disease that causes nonpulsatile tinnitus or other CVN dysfunction.2–4 Vascular plexuses around the ganglion of Scarpa may give rise to hemangiomas of the vestibular nerves or a rare thrombotic reactive process5,6 (Fig. 135.1). The similar cavernoma may produce CVN symptoms in the brain stem, especially following a thrombotic episode (Fig. 135.2G,H).
Clinical Presentation
Patients will generally present with hearing loss, tinnitus, and vertigo or balance problems similar to those shown with vestibular schwannoma. Since these lesions do not arise from the CVN or follow its course, the clinical picture is more variable than that of a schwannoma. Tinnitus may be pulse synchronous (Chapter 109). Nystagmus may be present on physical examination. Brain stem malformations may involve the sensory and neural tracts as well as other nearby cranial nerve nuclei.
Pathophysiology and Patterns of Disease
Vascular plexuses around the ganglion of Scarpa may be the origin of hemangiomas of the vestibular nerves.1–3 These lesions may compress or encase the CVN, thus interfering with its function. When large enough and extending over the facial canal, they might produce the same effects on the facial nerve. The lesions will enlarge typically along the CVN within surrounding leptomeninges and subarachnoid space. Compression with axonal injury and/or demyelination of the nerve will interfere with its function, causing hyperexcitability that results in vertigo, pulsatile tinnitus, and hearing loss.
A discussion of the flow dynamics, venous stenoses, aneurysms, and other complicating factors of pial–dural and other vascular malformations is beyond the scope of this resource. The basic physiology and growth and enlargement patterns of these vascular developmental lesions are discussed in Chapter 9.
Manifestations and Findings
Computed Tomography and Magnetic Resonance Imaging
The CT and MR appearance and growth patterns of the vascular malformations that might arise in this location are discussed in Chapter 9. Neither MRA nor even some current MDCT units are capable of producing definitive images of the angioarchitecture of arterialized lesions and/or acceptable assessment of flow dynamics as well as catheter angiography (Figs. 9.30, 109.3, and 135.2A–D).
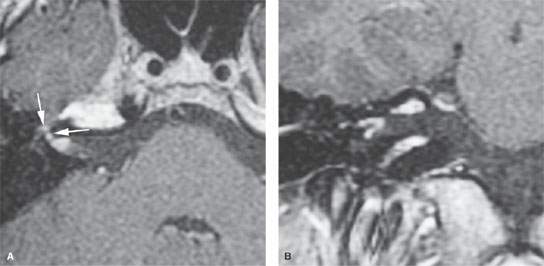
FIGURE 135.1. Masson vegetant intravascular hemangioendothelioma. On axial (A) and coronal (B) postcontrast T1-weighted magnetic resonance imaging, an enhancing mass is seen in the fundus of the internal auditory canal, and smaller nodular enhancement along the labyrinthine segment and anterior genu of the facial nerve is present (arrows in A), caused by intravascular papillary endothelial cell proliferation.
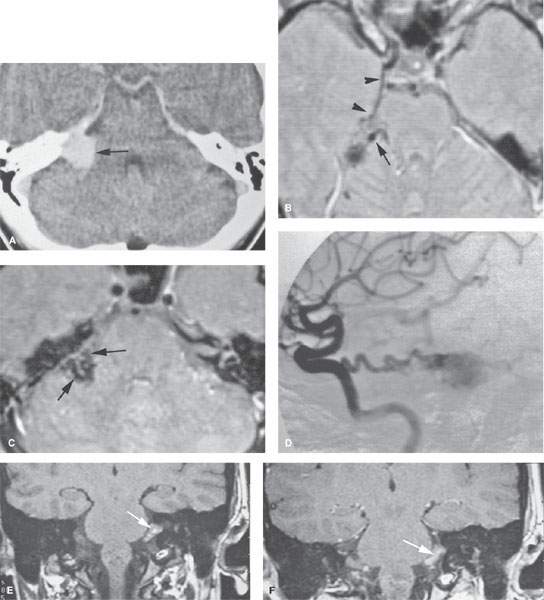
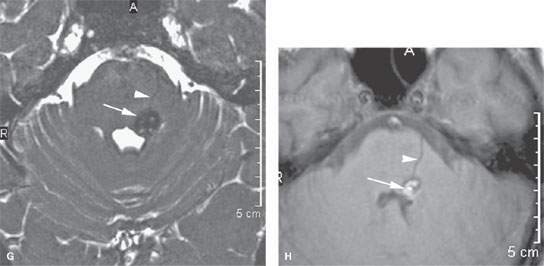
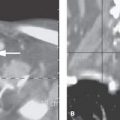
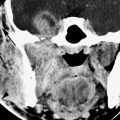
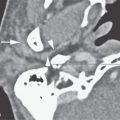
FIGURE 135.2. Three patients with vascular malformations along the course of the cochleovestibular nerve (CVN), all presenting with symptoms of CVN dysfunction. A–D: Patient 1. In (A), contrast-enhanced computed tomography (CT) shows an enhancing lesion in the cerebellopontine angle (CPA) cistern (arrow) with a possible dural base, suggesting the diagnosis of a meningioma. In (B) and (C), magnetic resonance imaging shows that the lesion consists of enlarged tortuous vessels, presumably fed by the artery of the free margin of the tentorium (arrow and arrowheads). In (D), catheter angiography confirms the presence of a high-flow dural arteriovenous malformation. E, F: Patient 2. In (E), non–contrast-enhanced T1 weighted image shows a lesion of unusual density and morphology in the CPA cistern. In (F), contrast-enhanced T1 weighted image at the same level shows it to enhance diffusely. Presumptive diagnosis was a slow-flow vascular malformation. It was followed for several years and did not change. G, H: Patient 3. There was an acute onset of vertigo, hearing loss, and hemifacial and hemibody sensory deficits to touch as well as pain and temperature. The three-dimensional steady state image (G) shows a thrombosed cavernoma (arrow) and related transpontine vessel (arrowhead); the same findings are evident on the non–contrast-enhanced T1-weighted image (H).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree