Colon and Appendix
William E. Brant
Sarah Erickson
Colon
Imaging Methods
The primary imaging methods for detection and characterization of colon abnormalities have continued to evolve over time. The persistently expanding availability of colonoscopy has continued to reduce the role of barium enema in imaging the colon. On the contrary, the use of CT to image the abdomen and pelvis continues to increase, making CT often the method of initial detection of colon disease. CT and MR (virtual) colonography challenge the role of traditional colonoscopy for polyp and cancer detection. Once a possible neoplastic lesion is discovered, however, colonoscopy or proctoscopy is needed for biopsy. The single-contrast barium enema is still occasionally used for the evaluation of colonic obstruction, fistulas, and in old, seriously ill or debilitated patients. The double-contrast (air-contrast) barium enema (Fig. 31.1) is favored for detection of small lesions (<1 cm), for documentation of inflammatory bowel disease, and for detailed imaging evaluation of the rectum (1,2). Colonoscopy is sporadically limited by occasional failure to reach the right colon. Then, barium enema or virtual colonoscopy is utilized to complete the examination. As elsewhere in the GI tract, CT complements colonoscopy and barium examinations by demonstrating intramural and extracolonic components of disease. It is excellent for demonstrating extrinsic inflammatory and neoplastic processes that affect the colon: abscesses, sinuses, and fistulas.
CT and MR imaging are utilized for initial staging of colorectal carcinoma. Both methods have limitations especially in determining involvement of regional lymph nodes. Significant improvements have been made in preoperative CT and MR staging with use of thin-slice MDCT, and high-resolution and diffusion-weighted MR techniques (3,4,5,6). PET-CT aids in the detection of metastatic disease to lymph nodes and distant metastases but is limited in assessment of local disease by physiologic and iatrogenic uptake of FDG by the colon (7). Transrectal US is more accurate than CT or MR in determining local tumor extent of rectal carcinomas and is used in the evaluation of other rectal and perirectal diseases (8).
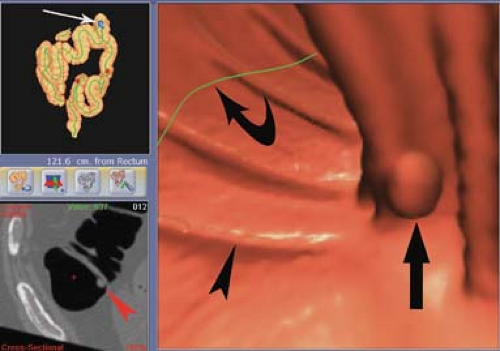
CT colonography (Fig. 31.2) is becoming a viable alternative to invasive colonoscopy to screen for colorectal cancer (9). The procedure begins with diligent bowel preparation identical to that used for invasive colonoscopy. A rectal tube is inserted and the colon is insufflated with carbon dioxide or room air. MDCT of the entire extent of the colon with the patient in supine position is obtained in a single breath-hold utilizing 1.25- to 2.5-mm collimation and a reconstruction interval of 1 mm. The scan is repeated with the patient in prone position. Commercially available software programs that provide endoluminal display and “fly-through” capabilities provide three-dimensional volume rendering image processing. Image viewing and interpretation is usually performed using both standard two-dimensional axial CT reconstructions and the three-dimensional volume-rendered images on a computer workstation. The role of virtual colonoscopy in imaging the colon and screening for colorectal cancer is still being debated, but it has been endorsed by a multisociety task force composed of the American Cancer Society, American College of Radiology, and the US Multi-Society Task Force on Colorectal Cancer for screening of average-risk adults (10). The role of virtual colonoscopy for colorectal cancer screening is likely to increase in the coming years.
MR colonography offers the advantage of screening for colorectal carcinoma without the use of ionizing radiation. Dark lumen MR colonography with filling and distention of the lumen with air, water, or other low signal intensity agents, fecal tagging agents, 3-Tesla magnets, and optimal pulse sequences show great promise (11). The same bowel preparation is needed as for colonoscopy or CT colonography. The bowel must be well distended with either bright lumen or dark lumen agents. Dark lumen agents are generally preferred because IV contrast agents can be effectively utilized. Limitations are expense and artifact such as from hip prostheses.
Anatomy
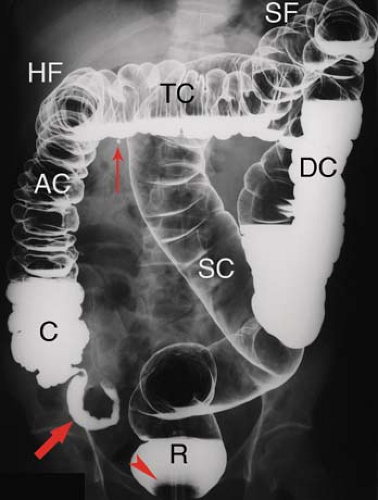
The large intestine consists of the cecum and appendix, colon, rectum, and anal canal. It is approximately 1.5 m in length from the ileum to the anus. The large intestine is characterized by the taenia coli, three longitudinal bands of muscle that traverse the colon shortening it to form haustra, the sacculations created by puckering of the bowel wall. The major functions of the large intestine are formation, transport, and evacuation of feces. These functions require mobility, absorption of water,
and secretion of mucus. Infrequent peristalsis transports feces from the ascending and transverse colon to the sigmoid colon where fecal material is stored until defecation. The cecum and ascending colon absorb water from the highly liquid material received from the ileum. Mucus secreted by mucosal goblet cells protects the mucosa from injury and is secreted in profuse amounts when the mucosa is irritated or injured. The cecum is the large blind pouch that extends below the level of the ileocecal valve. The cecum generally lies in the right iliac fossa but may be quite mobile. It is usually covered on all sides by peritoneum (intraperitoneal), but may be fixed extraperitoneally, covered only on its ventral surface by peritoneum. The appendix is a long worm-like tube that hangs from near the apex of the cecum. The ileocecal valve consists of two lips that project into the cecum forming a sometimes prominent mass (12). The ascending colon is extraperitoneal, lying in the anterior pararenal space, covered only on its ventral surface by peritoneum. The hepatic flexure forms two curves. The proximal, more posterior curve is closely related to the descending duodenum and right kidney. The more distal anterior curve is closely related to the gallbladder. The transverse colon is intraperitoneal and suspended from the transverse mesocolon that arises from the peritoneum covering the pancreas and sweeps transversely across the upper abdomen. The transverse mesocolon limits the superior extent of the small bowel loops. The splenic flexure is closely related to the tail of the pancreas and the caudal aspect of the spleen. The splenic flexure is anchored to the diaphragm by the phrenicocolic ligament, which serves as a boundary between disease processes of the left subphrenic space and the left paracolic gutter. The descending colon, like the ascending colon, is extraperitoneal within the anterior pararenal space and is covered by peritoneum only on its ventral surface. The sigmoid colon forms a redundant loop of variable length from the distal descending colon in the left iliac fossa to the rectum. The sigmoid colon is completely intraperitoneal and is suspended by the sigmoid mesocolon that allows considerable mobility. The sigmoid colon penetrates the peritoneum at the level of vertebrae S-2 to S-4 to continue as the extraperitoneal rectum. The rectum extends for approximately 12 cm in close relationship with the sacrum. Peritoneum forming the pouch of Douglas covers the ventral and the lateral aspects of the rectum. The anal canal is 3 to 4 cm long and is invested by the sphincter ani and levator ani muscles. A series of vertical folds form the rectal columns of Morgagni, beneath which are the veins that when dilated are hemorrhoids. The colon is recognized on imaging studies by its course, haustral markings, and fecal content. The thickness of the wall of the normal colon does not exceed 5 mm.
and secretion of mucus. Infrequent peristalsis transports feces from the ascending and transverse colon to the sigmoid colon where fecal material is stored until defecation. The cecum and ascending colon absorb water from the highly liquid material received from the ileum. Mucus secreted by mucosal goblet cells protects the mucosa from injury and is secreted in profuse amounts when the mucosa is irritated or injured. The cecum is the large blind pouch that extends below the level of the ileocecal valve. The cecum generally lies in the right iliac fossa but may be quite mobile. It is usually covered on all sides by peritoneum (intraperitoneal), but may be fixed extraperitoneally, covered only on its ventral surface by peritoneum. The appendix is a long worm-like tube that hangs from near the apex of the cecum. The ileocecal valve consists of two lips that project into the cecum forming a sometimes prominent mass (12). The ascending colon is extraperitoneal, lying in the anterior pararenal space, covered only on its ventral surface by peritoneum. The hepatic flexure forms two curves. The proximal, more posterior curve is closely related to the descending duodenum and right kidney. The more distal anterior curve is closely related to the gallbladder. The transverse colon is intraperitoneal and suspended from the transverse mesocolon that arises from the peritoneum covering the pancreas and sweeps transversely across the upper abdomen. The transverse mesocolon limits the superior extent of the small bowel loops. The splenic flexure is closely related to the tail of the pancreas and the caudal aspect of the spleen. The splenic flexure is anchored to the diaphragm by the phrenicocolic ligament, which serves as a boundary between disease processes of the left subphrenic space and the left paracolic gutter. The descending colon, like the ascending colon, is extraperitoneal within the anterior pararenal space and is covered by peritoneum only on its ventral surface. The sigmoid colon forms a redundant loop of variable length from the distal descending colon in the left iliac fossa to the rectum. The sigmoid colon is completely intraperitoneal and is suspended by the sigmoid mesocolon that allows considerable mobility. The sigmoid colon penetrates the peritoneum at the level of vertebrae S-2 to S-4 to continue as the extraperitoneal rectum. The rectum extends for approximately 12 cm in close relationship with the sacrum. Peritoneum forming the pouch of Douglas covers the ventral and the lateral aspects of the rectum. The anal canal is 3 to 4 cm long and is invested by the sphincter ani and levator ani muscles. A series of vertical folds form the rectal columns of Morgagni, beneath which are the veins that when dilated are hemorrhoids. The colon is recognized on imaging studies by its course, haustral markings, and fecal content. The thickness of the wall of the normal colon does not exceed 5 mm.
Colon Filling Defects/Mass Lesions
Filling defect refers to a radiolucency in a barium pool caused by a protruding mass lesion (9). On barium enema examinations, filling defects may be polyps, tumors, plaques, air bubbles, feces, mucus, or foreign objects. Polyps are protrusions from the mucosa that produce filling defects in pools of barium or are etched in white when coated by barium and outlined by air on double-contrast studies. Polyps may be pedunculated on a stalk (Fig. 31.3) or sessile. They may appear as “bowler hats” (Fig. 31.4) when viewed obliquely. The term “polyp” is generic for a protruding lesion and does not imply a histologic diagnosis. Air bubbles rise to the highest point of a contrast column (the “carpenter’s level sign”), but fecal material usually remains
dependent. Plaques are flat lesions that barely rise above the mucosal surface (13).
dependent. Plaques are flat lesions that barely rise above the mucosal surface (13).
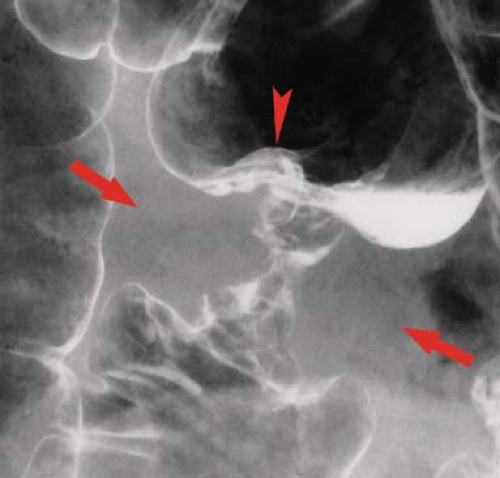
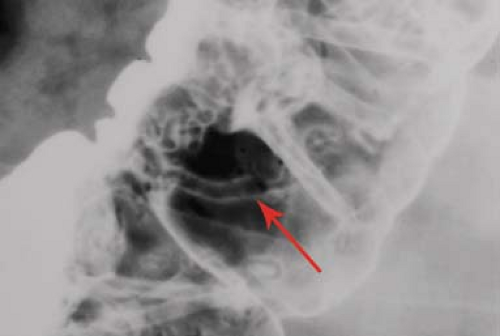
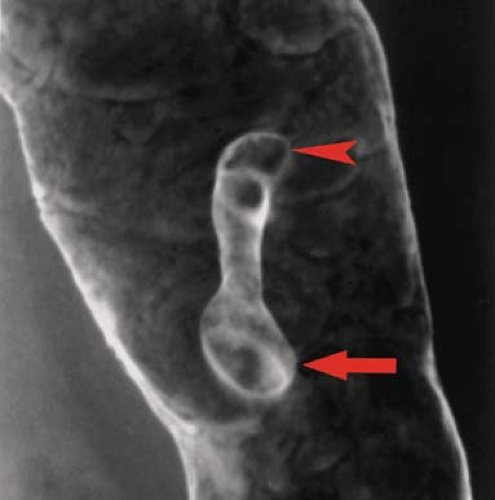 Figure 31.3. Pedunculated Polyp. Double-contrast barium enema demonstrates a long-stalked pedunculated polyp with a bulbous tip (arrow) arising (arrowhead) from the mucosa of the descending colon. |
Colorectal adenocarcinoma is the most common malignancy of the GI tract and the second most common malignant tumor in the United States. Approximately 50% arise in the rectum and rectosigmoid area. Another 25% occur in the sigmoid colon, and the remaining 25% are evenly distributed throughout the remainder of the colon. Nearly all cancers of the colon are adenocarcinomas arising from preexisting adenomas. Most tumors are annular constricting lesions, 2 to 6 cm in diameter, with raised everted edges and ulcerated mucosa (Fig. 31.5). Polypoid tumors are less common, some having the frond-like appearance of villous carcinoma (Fig. 31.6). Infiltrating scirrhous tumors, so common in gastric carcinoma, are rare in the large intestine, unless the patient has ulcerative colitis. The tumor spreads by direct invasion through the bowel wall into pericolonic fat (Fig. 31.7) and adjacent organs, lymphatic channels to regional nodes, and hematogenously through the portal veins to the liver and systemic circulation. Intraperitoneal seeding from a tumor that penetrates the colon wall may also occur. Obstruction is the most frequent complication. Other complications are uncommon but include perforation (Fig. 31.8), intussusception, abscess, and fistula formation. Up to 20% of patients have a second tumor of the large bowel at diagnosis, usually an adenoma or another carcinoma. Approximately 5% of patients will have a second colorectal carcinoma either simultaneously or subsequently
diagnosed. Patients with ulcerative colitis, Crohn disease, familial adenomatous polyposis syndrome, and Peutz–Jeghers syndrome are at increased risk of colon carcinoma.
diagnosed. Patients with ulcerative colitis, Crohn disease, familial adenomatous polyposis syndrome, and Peutz–Jeghers syndrome are at increased risk of colon carcinoma.
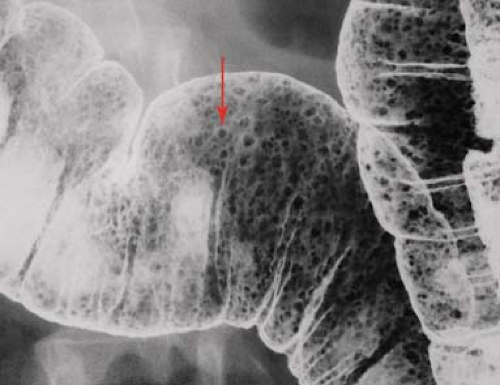
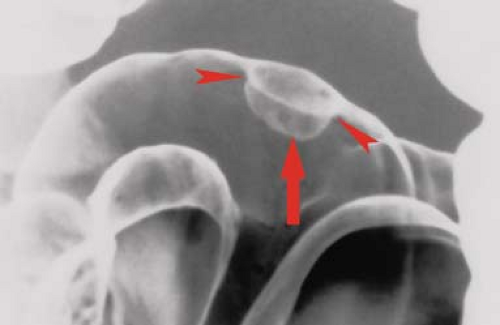 Figure 31.4. Bowler Hat Sign is produced by barium coating both the body of the polyp (arrow) and the recesses (arrowheads) between the base of the lesion and the normal colonic mucosa. |
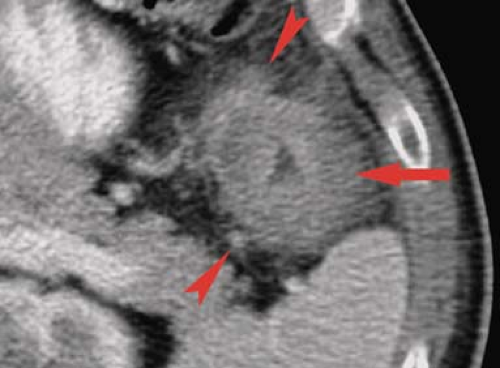
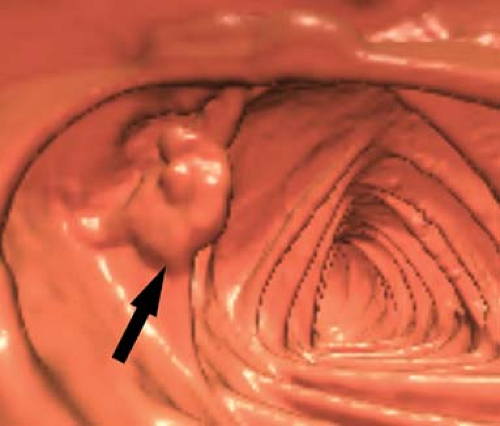 Figure 31.6. Colon Carcinoma—CT Colonography. A colon carcinoma (arrow) has the CT colonography appearance of a multinodular villous polyp. |
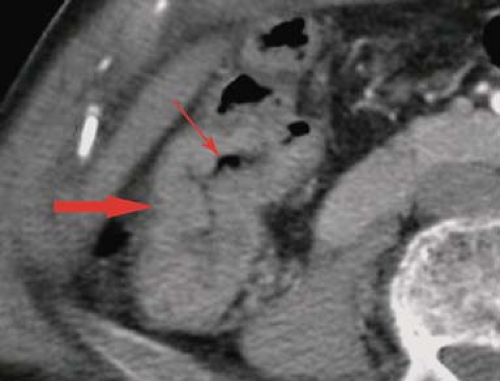
Local disease staging is best evaluated with transrectal or colonoscopic US. CT and MR are used for more advanced disease and to detect recurrence (14). Microscopic invasion through the bowel wall and tumor involvement of normal sized lymph nodes is not detected by CT or MR. Cross-sectional imaging findings include (1) polypoid primary tumor (usually >1 cm) (Fig. 31.6); (2) “apple-core lesions” with bulky, irregular thickening of the colon wall and irregular narrowing of the lumen (Fig. 31.9); (3) cystic, necrotic, and hemorrhagic areas within the tumor mass, especially when the tumor is large; (4) linear soft tissue stranding into the pericolonic fat often indicative of tumor extension through the bowel wall; (5) enlarged regional lymph nodes (>1 cm) representing lymphatic spread of tumor; and (6) distant metastases, especially in the liver (15). When tumors cause colonic obstruction, edema or ischemia may thicken the wall of the uninvolved colon proximal to the tumor.
Tumor recurrences are most common (1) at the operative site, near the bowel anastomosis; (2) in lymph nodes that drain the operative site; (3) in the peritoneal cavity; and (4) in the liver and distant organs. The entire abdominal cavity must be surveyed to detect tumor recurrence. CT, MR, and PET-CT are utilized to demonstrate response to therapy and tumor recurrence.
Polyps. A polyp is defined as a localized mass that projects from the mucosa into the lumen (13). Because the majority of colorectal cancers are believed to arise from preexisting adenomatous polyps, the detection of colon polyps is a major indication for colonoscopy and imaging studies of the colon. The following “rules of thumb” can be applied. Polyps less than 5 mm are almost all hyperplastic, with a risk of malignancy less than 0.5%. Polyps 5 to 10 mm size are 90% adenomas, with a risk of malignancy of 1%. Polyps 10 to 20 mm in size are usually adenomas, with a risk of malignancy of 10%. Polyps larger than 20 mm are 50% malignant.
Hyperplastic polyps are nonneoplastic mucosal proliferations. They are round and sessile. Nearly all are less than 5 mm in size.
Adenomatous polyps are distinctly premalignant and a major risk for development of colorectal carcinoma. Adenomatous polyps are neoplasms with a core of connective tissue. Approximately 5% to 10% of the population older than 40 years have adenomatous polyps.
Hamartomatous polyps (juvenile polyps) represent 1% of colon polyps. They are a common cause of rectal bleeding in children. The Peutz–Jeghers polyp is a type of hamartomatous polyp.
Inflammatory polyps are usually multiple and associated with inflammatory bowel disease (Fig. 31.10). They account for less than 0.5% of colorectal polyps.
Familial adenomatous polyposis syndrome is approximately two-thirds inherited and one-third spontaneous. The inheritance pattern is autosomal dominant with high penetrance. The polyps are tubulovillous adenomas, which usually are evident by age 20. Colorectal cancer will eventually develop in nearly all patients, and so, total colectomy with rectal mucosectomy and ileoanal pouch construction is the current recommended therapy. Polyps typically carpet the entire colon (Fig. 31.11). Patients are at risk for numerous extracolonic manifestations including carcinomas of the small bowel, thyroid carcinoma, and mesenteric fibromatosis. Patients with associated bone and skin abnormalities including cortical thickening of the ribs and long bones, osteomas of the skull, supernumerary teeth, exostoses of the mandible, and dermal fibromas; desmoids; and epidermal inclusion cysts have been diagnosed as Gardner syndrome. Those with associated tumors of the CNS have been grouped as Turcot syndrome. These are variations of the same disease.
Hamartomatous Polyposis Syndromes. Hamartomatous polyps are nonneoplastic growths with a smooth muscle core covered by mature glandular epithelium. The hamartomatous polyps associated with the various syndromes have minor histologic differences. These lesions carry no risk of malignant transformation. However, patients with the hamartomatous polyposis syndromes may also develop adenomatous polyps, which do carry a risk of malignancy.
Peutz–Jeghers syndrome predominantly involves the small bowel, but most cases have gastric and colon polyps as well. The condition is autosomal dominant with incomplete penetrance. Dark pigmented spots on the skin and the mucous membranes are characteristic. Risk of carcinoma arising from coexisting adenomatous polyps is 2% to 20%. Patients are also at risk for breast cancer, uterine and ovarian cancer, and early age cancer of the pancreas.
Cowden disease is a syndrome of multiple hamartomas including hamartomatous polyposis of the GI tract, with goiter and thyroid adenomas and increased risk of breast cancer and transitional cell carcinoma of the urinary tract. The syndrome is autosomal dominant and affects mainly Caucasians. All patients have mucocutaneous lesions with facial papules, oral papillomas, and palmoplantar keratoses.
Cronkhite–Canada syndrome is a disease of older patients with a mean age of onset of 60 years. Polyps are distributed throughout the stomach, small bowel, and colon. Associated skin findings include nail atrophy, brownish skin pigmentation, and alopecia. Patients present with watery diarrhea and protein-losing enteropathy.
Lymphoid hyperplasia may involve the colon. The normal lymphoid follicular pattern of diffuse tiny nodules 1 to 3 mm in diameter (Fig. 31.12) with characteristic umbilication is most common in the terminal ileum and cecum but may involve any portion of the colon. The nodular lymphoid hyperplasia pattern of diffuse nodules larger than 4 mm is associated with allergic, infectious, and inflammatory disorders.
Lymphoma. The colon is less commonly involved with lymphoma than the stomach or small bowel (16). Most are non-Hodgkin B-cell lymphoma. Involvement of the cecum or rectum is most common with anal and rectal lymphoma increasingly frequent in AIDS patients. Morphologic patterns include small to large nodules that may ulcerate, excavate, and perforate, and diffuse infiltration of the bowel wall resulting in bulbous folds and thickened bowel wall (Fig. 31.13). As in the small intestine, marked narrowing of the lumen is uncommon, and aneurysmal dilation occurs when transmural disease destroys innervation. The diffuse multinodular form may be
difficult to differentiate from nodular lymphoid hyperplasia. Lymphoma nodules vary in size although lymphoid hyperplasia nodules are uniform in size.
difficult to differentiate from nodular lymphoid hyperplasia. Lymphoma nodules vary in size although lymphoid hyperplasia nodules are uniform in size.
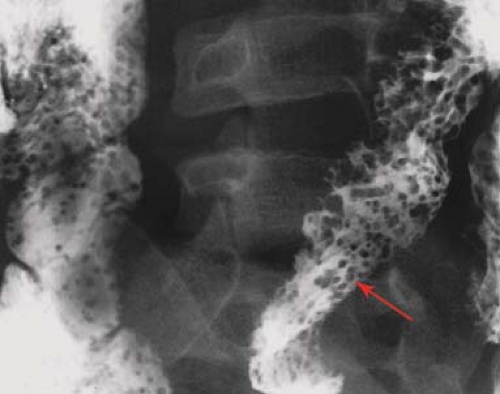 Figure 31.12. Nodular Lymphoid Hyperplasia. Single-contrast barium enema in a young patient with hypogammaglobulinemia shows numerous small nodules (arrow) throughout the colon. |
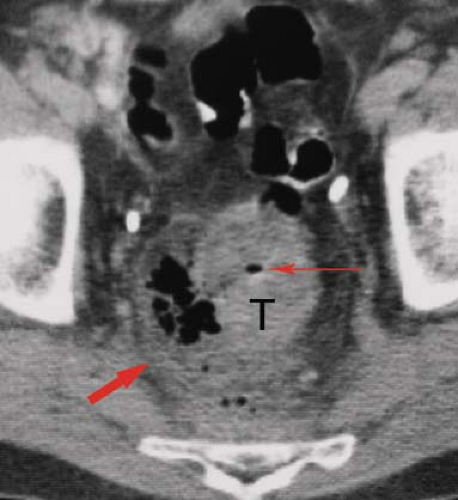
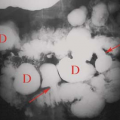
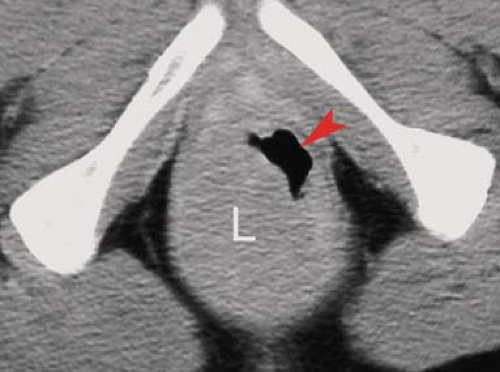 Figure 31.13. Rectal Lymphoma. CT demonstrates a prominent mass of lymphoma (L) that causes irregular narrowing of the lumen (arrowhead) of the rectum. Note the homogeneous attenuation of the lymphomatous mass. The CT appearance is indistinguishable from adenocarcinoma of the rectum.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|