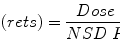, Foster D. Lasley2, Indra J. Das2, Marc S. Mendonca2 and Joseph R. Dynlacht2
(1)
Department of Radiation Oncology, CHRISTUS St. Patrick Regional Cancer Center, Lake Charles, LA, USA
(2)
Department of Radiation Oncology, Indiana University School of Medicine, Indianapolis, IN, USA
Radiosensitizers
Unlike chemotherapy, these drugs have little to no tumoricidal effect unless given in combination with radiation.
Halogenated Pyrimidines
Bromodeoxyuridine (BUdR) and Iododeoxyuridine (IUdR)
Nucleotide analogues that are taken up and incorporated into newly synthesized DNA.
Only cells with active DNA synthesis will incorporate these analogues.
DNA containing nucleotide analogues is more susceptible to strand breakage from ionizing radiation, therefore tumor is selectively radiosensitized.
Radioprotectors
These drugs are designed to selectively protect normal tissues from radiation damage.
Sulfhydryls (WR-series compounds)
Powerful free radical scavengers, developed during Cold War in anticipation of nuclear war.
Amifostine is the only FDA-approved radioprotector.
Administered 30 min prior to RT, greatly decreases mucositis and xerostomia.
Normal tissue selectivity is based on:
Slower penetration of tumors compared to well-vascularized normal tissues.
Alkaline phosphatase required to activate amifostine. Many tumors are deficient in this enzyme.
Can cause nausea and hypotension, and therefore is rarely used in modern clinical practice.
Oxygen Modifying Therapy
Hypoxia is a well known determinant of radioresistance. Many strategies have been developed to cope with hypoxia.
Direct Oxygen Modification:
Transfusions: RBCs, the oldest oxygen modifying therapy. The clinical evidence for radiosensitization by transfusion is mixed.
Hyperbaric Oxygen (HBO 2 ): Administered in a pressurized dive tank. Equipment and potential hazards make this option impractical for most medical centers.
Carbogen: A gas mixture of 95 % O2 and 5 % CO2, hyper-oxygenates tissues like HBO 2 but is easier to administer.
Nicotinamide: a vasodilator that decreases acute hypoxia.
Accelerated radiation with carbogen and nicotinamide (ARCON): A clinically relevant treatment regimen developed in the Netherlands.
Hypoxic Radiosensitizers:
These compounds increase the yield of DNA damage from irradiation under hypoxic conditions, much like oxygen itself.
Nitroimidazoles have a longer diffusion distance than oxygen and can therefore penetrate into a poorly vascularized tumor.
These include misonidazole, etanidazole, nimorazole and pimonidazole.
Radiolabeled nitroimidazoles can be used to image hypoxic tumor cells.
Effective use as radiosensitizers is limited by cumulative neurotoxicity that develops at concentrations required during fractionated RT.
Nimorazole is clinically used as a radiosensitizer in Danish head-and-neck trials.
Hypoxic Cytotoxins:
These compounds have inherent tumoricidal activity even in the absence of radiation.
Mitomycin C – chemotherapy drug, slightly more toxic to hypoxic cells. Very myelosuppressive, used routinely in anal cancer.
Tirapazamine – Hypoxia-specific toxin that works very well in mice but is more toxic in humans. Used in ongoing clinical trials for hypoxic H&N tumors.
Hypoxia Imaging
The “Gold Standard” used for invasive hypoxia detection is the oxygen probe. Use of these probes is painful for patients.
The non-invasive “gold standard” is hypoxia imaging with O-15 PET (radioactive oxygen).
O-15 has a half-life of 2 min so it has to be manufactured at or near the imaging site.
18F-MISO and 62Cu-ATSM are longer-lived positron emitters that accumulate in hypoxic cells and can be used for PET imaging.
It is theorized that hypoxia-specific sensitizers and cytotoxins will be more effective when coupled with hypoxia imaging.
Select out a group of highly hypoxic tumors that will respond better to hypoxia-specific agents.
This is not yet proven by clinical data (as of 2013), but there are ongoing trials.
Dose Reduction Factor and Enhancement Factor
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree