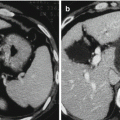
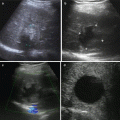
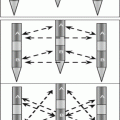
Fig. 8.1
sHCC treated by TACE+RFA
The effect of chemotherapeutic anticancer agents on cancer cells enhances the effect of hyperthermia [4]. TACE, being a regional treatment, can target undetected satellite lesions outside of the zone of RFA-induced necrosis. Moreover, disruption of intratumoral septa, which usually happens after TACE, facilitates heat distribution within the tumor, and intratumoral septa and fibrosis are considered to hamper heat diffusion within the tumor [4]. Sequential application of TACE and RFA is, therefore, increasingly being used in the treatment of HCC in patients with well-compensated liver disease. Recently, TACE-RFA has been reported to be an effective and safe treatment for HCC [5–11] (Table 8.1).
Table 8.1
Trials to compare TACE-RFA with RFA alone
Ref. | Design | Treatment | No. of patients | Recurrence-free survival rate | Overall survival rate | ||||
|---|---|---|---|---|---|---|---|---|---|
1 year (%) | 3 years | 5 years | 1 year (%) | 3 years | 5 years | ||||
Shibata et al. [5] | RCT | TACE-RFA | 46 | 71.30 | 48.80 % | NA | 100.00 | 84.8 % | NA |
RFA | 43 | 74.30 | 29.70 % | NA | 100.00 | 84.50 % | NA | ||
Morimoto et al. [6] | RCT | TACE-RFA | 19 | 67.00 | NA | NA | 100.00 | 93.00 % | NA |
RFA | 18 | 56.00 | 28.00 % | NA | 89.00 | 80.00 % | NA | ||
Peng et al. [7] | RCT | TACE-RFA | 94 | 79.4 | 60.6 % | NA | 92.6 | 66.6 % | NA |
RFA | 95 | 66.7 | 44.2 | NA | 85.3 | 59 % | NA | ||
Yang et al. [8] | Cohort | TACE-RFA | 24 | 29.00 | NA | NA | 68.00 | NA | NA |
RFA | 12 | 34.70 | NA | NA | 57.00 | NA | NA | ||
Shen et al. [9] | Cohort | TACE-RFA | 18 | 63.90 | 50.00 % | NA | 87.50 | 73.30 % | NA |
RFA | 16 | 30.00 | 18.70 % | NA | 52.20 | 20.40 % | NA | ||
The way of enhancing the efficacy of RFA by TACE is to decrease the blood flow of the tumor area to be ablated with subsequent decrease in heat loss by convection. Several studies have demonstrated the synergistic cytotoxic effects of TACE with RFA for the treatment of HCC, especially for medium-sized HCC (3–5 cm). The procedure for TACE-RFA is TACE first followed by RFA within 1 month. A recently published randomized controlled trial by Shibata et al. demonstrated a survival equality for combined TACE and RFA (n = 46) compared with RFA alone (n = 43) in patients with Child’s A or B cirrhosis and resectable HCC with ≤3 nodules smaller than 3 cm [5]. In this study, after treatment, the 1-, 2-, 3-, and 4-year rates of local tumor progression, overall survival, local progression-free survival, and recurrence-free survival were as follows: local tumor progression rates were 14.4 %, 17.6 %, 17.6 %, and 17.6 %, respectively, in the combined treatment group and 11.4 %, 14.4 %, 14.4 %, and 14.4 %, respectively, in the RFA group (P = 0.797). Overall survival rates were 100 %, 100 %, 84.8 %, and 72.7 %, respectively, in the combined treatment group and 100 %, 88.8 %, 84.5 %, and 74.0 %, respectively, in the RFA group (P = 0.515). Local progression-free survival rates were 84.6 %, 81.1 %, 69.7 %, and 55.8 %, respectively, in the combined treatment group and 88.4 %, 74.1 %, 74.1 %, and 61.7 %, respectively, in the RFA group (P = 0.934). Event-free survival rates were 71.3 %, 59.9 %, 48.8 %, and 36.6 %, respectively, in the combined treatment group and 74.3 %, 52.4 %, 29.7 %, and 29.7 %, respectively, in the RFA group (P = 0.365). In light of this study, the authors considered that combined RFA plus TACE and RFA alone have equivalent effectiveness for the treatment of small (≤3 cm) HCCs, and they thought the combination treatment may not be necessary. In the study, both treatment groups had low rates of major complications (2.2–2.3 %), and it was stated that treatment of HCC by combined TACE and RFA was safe. Morimoto et al. reported the midterm outcomes of a randomized controlled trial comparing the efficacy of TACE combined with RFA with RFA alone for the treatment of intermediate-sized HCC [6]. In the study, the authors randomly assigned 37 patients with solitary HCCs (diameter 3.1–5.0 cm in the greatest dimension) to two groups: the TACE combined with RFA (TACE-RFA) group and the RFA group. The results showed that technical success was achieved after 1.4 ± 0.5 RFA sessions in the RFA group and after 1.1 ± 0.2 RFA sessions in the TACE-RFA group (P = 0.01). The mean diameters of the longer and shorter axes of the RFA-induced ablated were 50 ± 8.0 and 41 ± 7.1 mm, respectively, in the RFA group and 58 ± 13.2 and 50 ± 11.3 mm, respectively, in the TACE-RFA group; the mean diameters of the shorter axes were significantly different (P = 0.012). The rates of local tumor progression at the end of the third year in the RFA and TACE-RFA groups were 39 and 6 %, respectively (P = 0.012). The 3-year survival rates of the patients in the RFA and TACE-RFA groups were 80 and 93 %, respectively (P = 0.369). The authors reported that TACE prior to RFA expanded the short axis of the ablated area and resulted in a more spherical ablated area. They postulated that a spherical ablated area was more effective than a nonspherical ablated area in ensuring local tumor control because a spherical ablated area was more likely to completely cover the target tumor. TACE-RFA was also a safe treatment in this study. The result showed that there were no severe side effects during the procedures in the study; however, six patients (five patients of the RFA group and one patient of the TACE-RFA group) experienced grade 1–2 pain lasting several hours. Major complications were not observed in the patients of the two groups. Minor complications, including asymptomatic right pleural effusion, were noted within 3 days of the procedures in two patients of the RFA group and one patient of the TACE-RFA group (RFA group vs. TACE-RFA group, P = 0.515). Recently, a randomized controlled trial published by Peng et al. demonstrated that the survival benefit for combined TACE and RFA (n = 94) was comparable with RFA alone (n = 95) in patients with Child’s A or B cirrhosis and resectable HCC with ≤3 nodules smaller than 7.0 cm [7]. In this study, after treatment, the 1-, 3-, and 4-year overall survival rates for the TACE-RFA group and the RFA group were 92.6 %, 66.6 %, and 61.8 % and 85.3 %, 59 %, and 45.0 %, respectively. The corresponding recurrence-free survival rates were 79.4 %, 60.6 %, and 54.8 % and 66.7 %, 44.2 %, and 38.9 %, respectively. Patients in the TACE-RFA group had better overall survival and recurrence-free survival than the RFA group (P = 0.002 and 0.009, respectively). After multivariate analysis, treatment allocation, tumor size, and tumor number were significant prognostic factors for overall survival, while treatment allocation and tumor number were significant prognostic factors for recurrence-free survival. There were no treatment-related deaths in this study. In light of this study, the role of combined TACE and RFA warrants careful consideration. The study provides evidence that altering the tumor microenvironment and supporting vasculature may help improve the efficacy of locoregional therapy in HCC.
Recently, some authors have used meta-analysis to verify the role of TACE combined with RFA in the treatment of HCC. In Yan’s studies, four randomized control trials were included into the meta-analysis [10]. Meta-analyses showed that the combination of RFA and TACE was associated with higher survival rates (1-year OR = 2.14, 95 % CI 1.57–2.91, P = 0.001; 3-year OR = 1.98, 95 % CI 1.28–3.07, P = 0.001; 5-year OR = 2.70, 95 % CI 1.42–.14, P = 0.003). They postulate that the combination of TACE with RFA can improve the overall survival rate and provide better prognosis for patients with HCC, but more randomized controlled trials using large sample size are needed to provide sufficient evidence. In another meta-analysis study, Wang et al. reported that there was no survival benefit from TACE combined with RFA in treating small HCC (≤3 cm) patients as compared with that of RFA alone [11]. The advantages of TACE-RFA may be as follows: TACE can block the hepatic arterial flow and contribute to the decrease in heat sink effects and the increase in the necrotic area induced by RFA, and the effect of anticancer agents on cancer cells may be enhanced by the hyperthermia. However, these advantages do not seem to have any indication according to Wang et al. meta-analysis for small HCC [11]. The reason for this may be that RFA has already achieved complete necrosis in >90 % in treating small HCC nodules, suggesting that adding TACE to RFA seems to be redundant in producing an assessed outcome. Schwartz and Weintraub considered that the clearest role for the combination of TACE and RFA is to increase the likelihood of complete destruction of HCC nodules that are in the range of 3–5 cm [12]. They also say that patients selected for combined TACE and RFA should have well-compensated cirrhosis and the rationale for using combined therapy is strongest for patients with uninodular HCC. Therefore, the role of TACE combined with RFA in the treatment of HCC needs further study.
TACE combined with RFA can also improve the efficacy of recurrent HCC after curative treatment [8, 13]. Yang et al. conducted a study to assess the efficacy and safety of RFA combined with TACE in recurrent HCC after hepatectomy and to compare its outcome with a single modality in recent year [8]. In this study, 103 patients with recurrent HCCs after hepatectomy who were excluded from repeat hepatectomy were allocated to three groups according to treatment modality. RFA was used as the sole first-line anticancer treatment in 37 patients (RFA group); TACE was used as the sole first-line anticancer treatment in 35 patients (TACE group). RFA followed by TACE was performed in 31 patients (combination group). There was no significant difference in clinical material between the three groups. The treatment success rate of the combination group was significantly higher than that of the TACE group (93.5 % vs. 68.6 %, P = 0.011). The intrahepatic recurrence rate of the combination group was significantly lower than that of the TACE group (20.7 % vs. 57.1 %, P = 0.002) and the RFA group (20.7 % vs. 43.2 %, P = 0.036). The overall 1-, 3-, and 5-year survival rates were 73.9 %, 51.1 %, and 28.0 %, respectively, in the RFA group; 65.8 %, 38.9 %, and 19.5 %, respectively, in the TACE group; and 88.5 %, 64.6 %, and 44.3 %, respectively, in the combination group. There was a significant difference in survival between the combination group and the TACE group (P = 0.028). Only one case of hemothorax was found in the combination group and recovered after percutaneous drainage. They thought that RFA combined with TACE was more effective in treating recurrent HCC after hepatectomy compared to single RFA or TACE treatment. This combination therapy can thus be a valuable choice of treatment for recurrent HCC. This study was limited by a possible selection bias resulting from the comparison of these nonrandomized groups and retrospective profile. Second, the number of TACE courses in each case was not strictly defined, because of repetitions planned on the basis of tumor response and patient tolerance. The best way to confirm this study’s result is to conduct randomized control trials. Peng et al. conducted a randomized study and try to figure out whether TACE combined sequentially with RFA is more effective than RFA alone for the treatment of HCC recurrence after curative treatment [13]. From January 2002 to December 2006, 139 patients with recurrent HCC ≤5 cm were randomized to receive either sequential TACE-RFA (n = 69) or RFA alone (n = 70). During follow-up, 32 patients in sequential treatment group and 35 patients in RFA group died. There was no significant difference between sequential treatment group and RFA group in terms of local tumor progression (1/69 vs. 2/70, P = 0.576). This prospective study showed that overall survival and recurrence-free survival for sequential TACE-RFA were significantly better than that for RFA alone (P = 0.037, P = 0.005, respectively). On subgroup analyses, the overall survival and recurrence-free survival for sequential TACE-RFA group were better than that for RFA group for patients with intervals of tumor recurrence from the initial treatment being ≤1 year (P = 0.004, P = 0.020, respectively) for tumors 3.1–5.0 cm in diameter (P = 0.002, P < 0.001, respectively). A logistic regression analysis showed that the interval of tumor recurrence from the initial treatment and treatment allocation were significant prognostic factors for overall survival, while the interval of tumor recurrence from the initial treatment, treatment allocation, and tumor size were significant prognostic factors for recurrence-free survival. According to this study, sequential TACE-RFA treatment has better efficacy than RFA for recurrent HCC ≤5 cm. For patients with 3–5 cm tumors or patients with interval of tumor recurrence from the initial treatment of ≤1 year, sequential TACE-RFA treatment should be recommended.
8.3 Combined with Percutaneous Ethanol Injection (PEI)
PEI had been utilized in the treatment of HCC for a long time. It usually requires to be repeated several times and with the disadvantages of long treatment cycle, high local recurrence rate, etc. RFA-PEI is also an effective combined treatment for HCC. Studies have demonstrated that RFA-PEI is able to achieve a larger ablative zone than RFA, which will lead to lower local recurrence rate and better survivals [14] (Figs. 8.2 and 8.3).
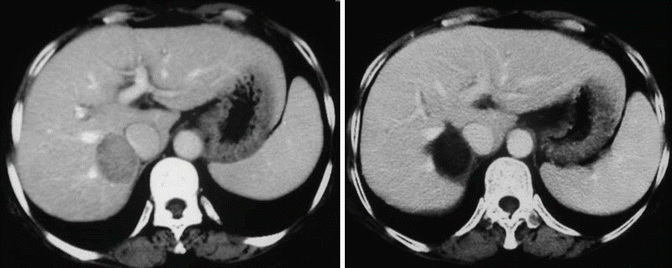
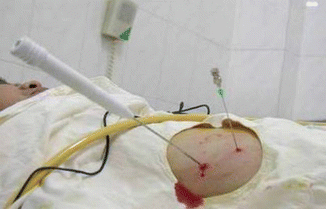

Fig. 8.2
sHCC treated by PEI+RFA

Fig. 8.3
Procedure for PEI+RFA
The procedure for RFA-PEI is RFA needle and PEI needle are inserted into the tumor at the same time, absolute alcohol is injected first, and then RFA is performed minutes after PEI. During RFA combined with PEI therapy (PEI followed by RFA), the injected ethanol embolizes vessels ≤5 mm, so that blood infusion is reduced. Meanwhile, the ethanol can disperse to area which RFA failed to reach, such as perivascular tumors. In this way, the ablative effect is enhanced. In Zhang et al. RCT, 133 patients were randomly assigned to receive RFA-PEI (n = 66) or RFA alone (n = 67) [14]. The 5-year overall survival rates for the RFA-PEI group and the RFA alone group were 49.3 % and 35.9 %, respectively. The RFA-PEI offered significant survival advantage over RFA alone for patients with tumors of 3.1–5.0 cm in diameter but not for those with tumors equal or less than 3.0 cm in diameter, or for those with tumors 5.1–7.0 cm in diameter. Moreover, some reports have suggested that RFA combined with injection of cytotoxic drugs will improve the efficacy although this remains to be proved.
PEI can be useful when tumors are in close vicinity (<1 cm) to vital structures, including biliary ducts, stomach, intestinal loops, and kidney, and whose location makes them difficult to treat with thermal ablative techniques. In addition, PEI is useful for lesions that remain undetected by ultrasound, such as masses in the hepatic dome or small nodules, or when the tumor is located subcapsularly or exophytically or surrounded by major vessels. The efficacy of RFA in HCC can be improved if combined with PEI. The combination of RFA and PEI was shown to be more effective than RFA alone for high-risk locations [15, 16].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree