Cell surface receptor
Cell surface receptor ligand
Therapeutic agonist/antagonist examples
Costimulatory
CD40
CD40L
CP-870,893 (Pfizer), dacetuzumab (Seattle Genetics)
CD134/OX40
CD252/OX40L
MEDI0562, MEDI6469, MEDI6383 (AstraZeneca)
GITR
GITRL
TRX518 (GITR Incorporated)
CD137/4-1BB
CD137L
PF-05082566 (Pfizer), lipocalin (Pieris Pharmaceuticals), urelumab (Bristol-Myers Squibb)
Inhibitory
CD152/CTLA4
CD80, CD86
Ipilimumab (Bristol-Myers Squibb), tremelimumab (AstraZeneca)
LAG3
MHC II
BMS-986016 (Bristol-Myers Squibb), IMP321 (Immuntep)
PD-1
PD-L1, PD-L2
Nivolumab (Bristol-Myers Squibb), pembrolizumab (Merck), pidilizumab (Cure Tech), AMP-224, AMP-514 (Amplimmune)
TIM-3
Galectin-9, HMGB1, PS, CEACAM-1
Anti-TIM-3 (Tesaro)
Several investigations combining radiotherapy and lymphocyte receptor modulators in preclinical models have been reported. Many of these studies use similar immunologic experimental methods. For readers unfamiliar with these methods, they are explained briefly here. The reader is also referred to several recent reviews on the immunologic effects of radiation therapy for further understanding of the effect of radiation on the immune system, in the absence of lymphocyte receptor modulators [11, 12].
As the target for experimental manipulation is the immune system, most studies are performed in vivo, rather than in vitro. For this reason, the models must use immunocompetent syngeneic species -specific (often murine) tumor grafts, rather than xenografts from human tumors in immunocompromised hosts. Investigators have studied tumor grafts placed subcutaneously or intradermally (on the flank or hind limb) and orthotopically (in the organ of tissue origin, such as the breast, brain, or skin). Tumor size, tumor growth delay, tumor response, metastasis, and overall survival are often the simplest measures of treatment effect. To demonstrate immune-mediated response to cancer distant from the radiotherapy target, some models incorporate two tumors, where one is irradiated and the other is not irradiated. This allows for demonstration of an abscopal effect (effect of radiation away from the target of radiotherapy) [13].
Immunologic response to tumor, radiotherapy , and lymphocyte receptor modulation can also be characterized at the treated tumor or in the peripheral lymphoid organs. Often immune cell populations (lymphocytes , myeloid cells , macrophages ) are characterized based on cell surface markers (of differentiation, activation, exhaustion, etc.) in different anatomic compartments (infiltrating the tumor , draining the lymph node basin, spleen, etc.). The dependency of the treatment effect on specific immune cell populations can be interrogated by performing experiments in animal models deficient for immune function (through genetic knockout) or through depletion of immune cell populations by neutralizing monoclonal antibodies against cell surface markers (CD4, CD8, etc.) and ligands (PD-L1 , TIM-3 , etc.). Determining whether immune cells recognize tumor-specific antigens can be carried out using ex vivo assays to determine if lymphocytes can kill tumor cells or if they elaborate cytokines such as interferon gamma in response to tumor antigens. Finally, immunologic memory can be tested after complete tumor regression by rechallenging the host with the tumor graft and assessing for the presence or absence of tumor growth. Similarly, immune cells from hosts with complete tumor regression can be adoptively transferred to naïve, tumor-bearing animals to assess for antitumor properties of the transferred immune cells .
Combinations of Costimulatory Receptor Modulation and Radiotherapy
CD137/4-1BB
CD137 or 4-1BB is a member of the tumor necrosis factor receptor (TNFR) superfamily and is expressed on T cells and other immune subsets following activation. Ligation of the receptor via its ligand or agonist antibodies results in enhanced T cell proliferation and production of cytokines . CD137 activation has also been shown to provide a strong survival signal for CD8 T cells via upregulation of antiapoptotic pathways [14]. In 2006, investigators first reported on the combination of 4-1BB agonism (with a monoclonal antibody, BMS-469492) and radiotherapy (5–15 Gy/1 fraction or 40 Gy/10 fractions) in a preclinical breast (EMT6) and lung (M109) cancer model. The authors found that 4-1BB agonism could effectively delay the growth of tumors in the breast cancer model, but not the lung cancer model. In the breast cancer model, when treated with the combination of single-dose or fractionated radiotherapy followed by 4-1BB agonism, investigators observed a delay in tumor growth significantly longer than either therapy when given alone. In the lung cancer model, only the highest single dose of radiotherapy (15 Gy), but not fractionated treatment, yielded a significant delay in tumor growth compared to either therapy given alone. The lung cancer cell line was found to have high basal expression of 4-1BBL which could not be increased by irradiation, while the breast cancer cell line had low basal expression of 4-1BBL, which could be increased by irradiation [15]. This suggests that the expression of 4-1BB ligand may be a good biomarker for combining RT with 4-1BB agonism.
In a preclinical orthotopic model of glioma using the GL261 cell line, investigators observed that the 4-1BB agonist antibody (BMS-469492) in combination with whole-head radiotherapy (8 Gy/2 fractions) yielded significantly longer survival rates than either treatment alone. Of the long-term survivors treated with radiotherapy alone (n = 2) or in combination with BMS-469492 (n = 6), 50 % and 83 % demonstrated no evidence of tumor regrowth after tumor rechallenge, respectively. All had pathologic complete response in the brain. When examining the tumor-infiltrating lymphocytes, significantly higher numbers of CD8 and CD4 lymphocytes were noted in the group treated with radiotherapy alone compared to the untreated control group, and even higher numbers were observed in those treated with 4-1BB agonism and radiotherapy. Finally, the production of interferon gamma, indicative of T cell effector function, in a tumor-specific manner by splenocytes was greatest in the group treated with 4-1BB agonism and radiotherapy [16].
CD134/OX40
CD134 or OX40 is another member of the TNFR superfamily expressed on activated CD4 and CD8 T cells as well as neutrophils, DCs, and Treg cells. The natural ligand (OX40L) is found on APCs as well as activated T cells. Engagement of OX40 promotes T cell activation, maturation, survival, and cytokine production [17]. Investigators have also observed that single-dose radiotherapy (20 Gy) followed by OX40 agonism (with a monoclonal antibody clone OX86) increased the rate of cure in a preclinical lung (Lewis lung carcinoma ) model, compared to either treatment alone. This effect was found to be dependent on CD8 lymphocytes, but not CD4 lymphocytes or natural killer cells. The combination of OX40 agonism and radiotherapy significantly increased the proportion of CD8 lymphocytes in the draining lymph node compared to either treatment alone. The CD8 lymphocytes had the ability to kill the lung cancer cell line in an antigen-specific manner. Finally, the combination of OX40 agonism and radiotherapy was found to yield immunologic memory and tumor rejection after rechallenge, compared to animals not previously treated [18].
Other investigators found that in a preclinical model of lung cancer (Lewis lung carcinoma , LLC), radiotherapy (60 Gy/3 fractions) followed by OX40 agonism (starting one day after the first fraction of radiotherapy) yielded significantly longer survival compared to either treatment alone. Tumor rechallenge after combination radiotherapy and OX40 agonism demonstrated immunologic memory [19].
GITR
Glucocorticoid-induced TNFR family-related (GITR) gene is expressed on CD4 and CD8 T cells and is upregulated after activation. Similar to other TNFR family members, ligation with its natural ligand (GITRL) expressed on activated APCs and endothelial cells (EC) results in enhanced T cell proliferation, survival, and effector function [20]. Investigators explored the effect of radiotherapy (30 Gy/1 fraction) with or without GITR agonism using a monoclonal antibody (DTA-1) in a lung carcinoma (Lewis lung carcinoma , LLC) model. The authors observed that irradiation of LLC significantly delayed tumor growth and increased survival, compared to no irradiation. Depletion of CD8 lymphocytes significantly decreased the tumor growth delay and survival suggesting that the effect is CD8 dependent. When GITR agonism and radiotherapy were combined, there was a nonsignificant tumor growth delay greater than either treatment alone, but no association with longer survival [21].
CD40
CD40 is another member of the TNFR superfamily constitutively expressed on APCs, and its ligation results in promotion of functional maturation with enhanced antigen presentation and cytokine production resulting in increased activation of T cells [22]. In 2003, investigators reported on studies of two syngeneic models of B cell lymphoma (A31 and BCL1) treated with total body irradiation (TBI, 2–8 Gy/1 fraction) for systemic lymphoma and/or costimulation by CD40 agonist monoclonal antibody 4 h after irradiation. The investigators observed a significant increase in survival with the combination of TBI and CD40 agonism, compared to either treatment alone. However, the effect was dependent on the dose of TBI used; 5 Gy yielded the highest proportion of survivors, with higher or lower doses of radiation proving inferior. The authors found that a wide range of doses of the CD40 agonist were effective at promoting survival, but that other B cell-depleting antibodies (against CD19, MHC II, CD22) did not yield the same effect as the CD40 agonist suggesting that the CD40 antibody is not acting by simply depleting B cells. In vitro analyses of apoptosis and clonogenic survival suggest that CD40 agonism did not increase the cellular radiosensitivity of the lymphoma cell lines. Interestingly, in an experiment where variable numbers of lymphoma cells were inoculated, the authors observed that a minimum amount of lymphoma cells must be treated to yield long-term immunity, again suggesting that CD40 is not a general sensitizer to radiation . By tracking the number of lymphoma cells present after combination treatment, investigators found that TBI alone yielded a dose-dependent decrease in the number of lymphoma cells, which regrew in the absence of CD40 agonism. The combination of TBI and CD40 agonism led to a two-phase (early and late) pattern of lymphoma regression. Importantly, a significant increase in CD8 cells was noted in animals treated with 5 Gy of TBI and CD40 agonism compared to those given 5 Gy of TBI alone. However, this was not observed with higher (8 Gy) or lower (2 Gy) doses of radiation , or in animals not bearing lymphoma, or with the use of other monoclonal antibodies. The authors observed that CD8 cells in the group receiving CD40 agonism and TBI had a significantly greater lymphoma-specific cytotoxic activity. In addition, CD8, but not CD4, lymphocyte depletion abrogated the therapeutic effect of TBI and CD40 agonism. In long-term survivors of the CD40 agonism and TBI treatment, rechallenge with lymphoma cells demonstrated immunologic memory in 80 % of the treated animals. Finally, adoptive transfer of T cells from survivors of the CD40 agonism and TBI combination to untreated lymphoma-bearing animals significantly increased the duration of survival in the recipient mice [23].
Combinations of Multiple Costimulatory Receptor Modulators and Radiotherapy
In 2012, investigators explored the combination of targeting multiple costimulation modulators in combination with radiotherapy in preclinical models. Using two triple-negative (estrogen/progesterone/Her-2/neu receptor negative) breast cancer cell (4T1.2 and AT-3) models, the authors explored the effect of 4-1BB and CD40 agonism alone, in combination, or immediately after radiotherapy (12 Gy/1 fraction). It was observed that 4-1BB agonism alone, or in combination with CD40 agonism, significantly delayed tumor growth compared to control. Notably, CD40 agonism alone did not significantly delay tumor growth. Likewise, when given radiotherapy, 4-1BB agonism alone, or in combination with CD40 agonism, significantly delayed tumor growth. This effect was not observed with CD40 agonism after radiotherapy. In the 4T1.2 cell line, tumor cure was observed with radiotherapy or in combination with CD40 and 4-1BB agonism; tumor cure occurred most often in the group receiving the combination of CD40 and 4-1BB agonism and radiotherapy. The antitumor effect was noted to be dependent on CD4, CD8, and natural killer cells. Moreover, rechallenge of the host with a tumor demonstrated immunologic memory. The authors hypothesized that the differences in response to the combination of immunotherapy and radiotherapy in the two cell lines were associated with 4T1.2 tumors supporting a necrotic core and undergoing an immunogenic, non-apoptotic death after radiotherapy, while AT-3 cells expressed PD-L1 , possibly conferring resistance to the combination of costimulation and radiotherapy. The authors conducted further experiments to explore ways to overcome resistance (described further below) [24].
Combinations of Inhibitory Receptor Modulation and Radiotherapy
CTLA4
APCs present antigen in the context of MHC to a specific T cell receptor on the surface of T cells. However, for resulting T cell activation, costimulation is required by a variety of other cell surface receptors including CD28 on the T cell interacting with CD80/B7.1 and CD86 B7.2 on APCs. CTLA4 is a member of the CD28 family of receptors and is upregulated on activated T cells . CTLA4 has a higher affinity for CD80/CD86 than the costimulatory receptor CD28 and can therefore competitively bind ligand more avidly than CD28. Through this mechanism, it acts as a negative feedback loop for T cell activation after TCR stimulation. CTLA4 is also expressed constitutively at high levels on Treg cells and is important for their suppressive functions. The administration of anti-CTLA4 antibodies results in blockade of inhibitory signals as well as direct depletion of Treg cells resulting in immune activation [25].
In 2005, investigators reported on the effects of radiotherapy (12 Gy/1 fraction or 24 Gy/2 fractions 48 h apart) alone or followed by CTLA4 blockade with the antibody 9H10 in a breast cancer (4T1) model. The growth of implanted 4T1 tumors was significantly delayed only in animals treated with radiotherapy, with or without 9H10, compared to untreated controls. Treatment with 9H10 alone did not delay tumor growth. Moreover, radiotherapy or CTLA4 blockade did not significantly increase survival compared to the group that did not receive treatment. However, the combination of CTLA4 blockade and RT did significantly increase survival compared to the untreated control group. Compared to untreated controls, a significantly lower number of lung metastases were observed only with the combination of CTLA4 blockade and radiotherapy, but not either treatment alone. This effect was abrogated with CD8 lymphocyte depletion, but not CD4 lymphocyte depletion . The authors further demonstrated that a higher total dose of radiation (24 Gy/2 fractions) yielded a 57 % rate of complete regression of the primary tumor, which was not observed for the lower dose of radiation (12 Gy/1 fraction). Despite improvement in primary tumor control with a higher dose of radiation, similar to prior experiments, the combination of CTLA4 blockade with 9H10 and radiotherapy yielded significantly longer survival than either treatment alone or no treatment at all. In the group with long-term survival, tumor rechallenge demonstrated protective immunity with 4T1-specific cytolytic activity in the spleen [26].
A subsequent study from the same group investigated the effect of single-dose (20 Gy/1) or fractionated radiotherapy (30 Gy/5 fractions or 24 Gy/3 fractions) with or without concurrent or subsequent CTLA4 blockade with a monoclonal antibody (9H10) in breast cancer (TSA) or colon cancer (MCA38) models. Using a two-tumor model where tumors were implanted on each flank of the mice but only one tumor was irradiated (as illustrated in Fig. 7.1), the authors observed that 9H10 alone had no effect on tumor growth compared to untreated controls. Radiotherapy alone caused tumor growth delay of the irradiated tumor of a similar magnitude across the three-dose schedules, but no growth delay in the unirradiated distant tumor . The combination of CTLA4 blockade and fractionated radiotherapy (but not single-dose radiotherapy) was associated with regression of both irradiated and unirradiated tumors demonstrating an abscopal effect . The effect was greatest in the 24 Gy/3 fraction regimen. The investigators further explored the effect of delaying the start of immunotherapy after initiating radiotherapy. They found that the longest delay between immunotherapy and radiotherapy was associated with the most rapid rate of tumor regression. Examination of the unirradiated tumors in the group receiving 9H10 and radiotherapy (24 Gy/3 fractions) demonstrated a significantly greater number of tumor-infiltrating CD4 and CD8 lymphocytes , compared to either treatment alone. Finally, the ex vivo tumor-specific production of interferon gamma by splenocytes was greatest in animals exhibiting rejection of the unirradiated tumor [27].


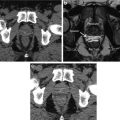
Fig. 7.1
Two-tumor model for the assessment of the abscopal effect . In this model, bilateral tumor grafts are placed, typically with one tumor being smaller than the other (a). Radiotherapy is administered to the larger of the two tumors (b), and the immune response in the tumors can be assessed after treatment (c). The unirradiated tumor is observed for abscopal response, or response away from the target of radiotherapy (d). This effect is thought to be immune mediated
Another group of investigators examined the effects of combining radiotherapy (2–30 Gy/1 fraction) with or without CTLA4 blockade using a monoclonal antibody (9H10) in a lung carcinoma (Lewis lung carcinoma , LLC) model. The authors assessed secretion of HMGB1 , a protein released after immunogenic cell death, after LLC cell irradiation in vitro. They noted no difference in HMGB1 levels between cells irradiated with 2 Gy and those not irradiated. However, cells irradiated with 6 or 30 Gy released threefold more HMGB1 than those not irradiated. The authors then carried out in vivo experiments, observing that radiotherapy (30 Gy/1 fraction) significantly delayed tumor growth and increased overall survival, compared to no radiotherapy. CD8 lymphocyte depletion significantly decreased the tumor growth delay and survival. CTLA4 blockade and radiotherapy significantly increased tumor growth delay and overall survival, compared to radiation or CTLA4 blockade alone [21].
Subsequently, other investigators studied radiotherapy (5–15 Gy/1–3 fractions) in combination with CTLA4 blockade with a monoclonal antibody in a mesothelioma model (AB12) in immunocompetent or immunodeficient (nonobese diabetic/severe combined immunodeficient, NOD/SCID) hosts. Using a two-tumor model , the authors found that irradiating one tumor leads to significant delay in growth of the irradiated tumor, as well as the unirradiated tumor. However, immunodeficient (NOD/SCID) hosts did not demonstrate delay in growth of the unirradiated tumor suggesting that the adaptive immune system is important in controlling the growth of the unirradiated tumors. The combination of CTLA4 blockade and radiotherapy delayed irradiated and unirradiated tumor growth significantly longer than either treatment alone. These same observations were made whether the irradiated tumor and unirradiated tumor were implanted synchronously or metachronously. Assessment of immune infiltrates in the tumor, draining lymph nodes , and spleen 10 days after treatment revealed significantly higher levels of activated (ICOS+) and proliferating (Ki67+) CD4 and CD8 lymphocytes , as well as dendritic cells in the draining lymph nodes (but not the spleen) of hosts with irradiated tumors, compared to control tumors. Significantly more CD8 T cells were noted in irradiated tumors in the group treated with CTLA4 blockade and radiotherapy, compared to those treated with either treatment alone. In the group treated with CTLA4 blockade and radiotherapy, a significant increase in the number of activated (but not total) CD4 and CD8 T cells in the spleen was observed compared to the group treated with radiotherapy alone. Finally, compared with irradiated or unirradiated tumors treated with radiotherapy alone, those treated with CTLA4 blockade and radiotherapy demonstrated an increase in the expression of pro-inflammatory markers including interferon gamma, perforin, IP-10, TNF alpha, granzyme B, ICOS, IL-4, IL-12, IL-12p70, IL-5, IL-6, IL-17A, and MCP-1 [28].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree