Author
No. of patients
Approach
No. of tumors
Tumor size (cm)
Mortality (%)
Major complications (%) (per patient/per session)
PHC
Mets
Liang et al. [2]
1,136
PC
1,412
516
3.4 (0.7–8)
0.2
2.6/N/A
Livraghi et al. [4]
736
PC(554)
Total 1,037
0.5–10
0
2.9/N/A
LS(137)
OS (45)
Ding et al. [5]
556
PC
Total 1,090
2.3(0.5–6.0)
0.4
3.6/N/A
Wang et al. [6]
693
PC
898
2.5
0.4
3.9/N/A
6.2 The Spectrum of Complications
6.2.1 Major Complications
According to the recent proposal of the International Working Group of Image-Guided Tumor Ablation in 2009, the definition of death is self-explanatory and should be reported on a per-patient basis. Any patient death within 30 days after ablation should be addressed. The definition of major complication is an event that leads to substantial morbidity, increasing the level of care, or results in hospital admission or substantially lengthened hospital stay. This includes any case in which a blood transfusion or interventional drainage procedure is required. All other complications are considered minor [7].
6.2.1.1 Patient Mortality
Death within 30 days always follows major complications after ablation. The mortality rates ranged from 0.2 to 0.4 %. Our latest results showed five cases (0.4 %) of death. The possible causes are massive arterial bleeding, liver failure, cardiopulmonary complications, multiple organ failure, hepatorenal syndrome, ischemic bowel due to poor physical status, pulmonary embolism caused by detachment of inferior vena cava thrombus, upper gastrointestinal hemorrhage due to severe portal hypertension, or other existing basic illnesses [2, 8, 9]. Careful selection of patients should be highlighted before any procedure. To avoid the possible risks of death, ablation should be carefully weighted on those with severe liver dysfunction, poor physical status, advanced stage diseases, cardiopulmonary diseases, and other serious underlying diseases. An IR should grasp the whole spectrum of major complications, detect complications, and give appropriate treatment as early as possible.
6.2.1.2 Intraperitoneal Hemorrhage
Bleeding is a frequent complication of MWA for liver tumors due to the coagulopathy associated with the underlying liver cirrhosis. However, intraperitoneal hemorrhage is rare, with an incidence rate ranging from 0.1 to 0.4 %. The possible causes are as follows: direct injury to the intrahepatic vessel due to electrode placement, coagulation dysfunction due to cirrhosis, needle-tract bleeding or tumor rupture, delayed hemorrhage of ruptured pseudoaneurysm, etc. [5, 10, 11]. Bleeding is more likely due to direct mechanical damage to the vessel than thermal injury. Our latest results showed only one case of hepatic artery injury hemorrhage due to noncooperation of the patient. Every effort should be made to prevent such serious complication, particularly in cases of superficial and large liver tumors [12]. Our tips are as follows: (1) coagulation dysfunction should be corrected in an acceptable level before any interventional procedure; (2) it is important to select an appropriate needle path that avoids vital structures and traverses sufficient normal hepatic parenchyma; (3) real-time monitoring of the entire procedure, including microwave (MW) electrode placement, is crucial for avoiding such complications; and (4) sufficient ablation of needle track, minimum needle puncture into tumor issues, and cooperative breath from patients or even sedation anesthesia will be necessary.
Bleeding usually occurs during the procedure or within 6 h after therapy. Peripheral hepatic effusion and its amount should be checked during or immediately after the procedure. Patients’ vital signs and laboratory tests after the ablation should be closely monitored for early detection of this life-threatening complication, especially for those with severe cirrhosis and bleeding tendency. Conservative treatment such as thrombin for injection or blood transfusion will be helpful to most cases of venous hemorrhage. But for intractable arterial hemorrhage, transarterial embolization or open surgery will be necessary.
6.2.1.3 Hepatic Abscess
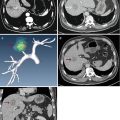
Hepatic abscess is a rather serious complication after thermal ablation, if not appropriately managed, which may cause sepsis, septic shock, multiorgan failure, or even death. The incidence rate was 0.1–0.4 %. Bilioenteric anastomosis was reported as an independent risk factor in the formation of liver abscess [13] (Fig. 6.1). Other abnormalities include cholangitis, diabetes mellitus, retained iodized oil in the ablation region, an internally cooled electrode with great power (200 W), and absorption of massive necrotic material after one-time ablation of large lesions [14–16]. Yu et al. concluded that intrahepatic cholangiocarcinoma and possible MWA also contributed to the high incidence of liver abscess [17]. In our latest study, although the metastatic group has a higher incidence of cholangiojejunostomy history, there was no significant difference in abscess rates between the two groups (P = 0.08). This is probably because of the wide use of intestinal preparation and prophylactic administration of high-efficiency antibiotic in the case with history of bilioenteric anastomosis or pathologic diagnosis of intrahepatic cholangiocarcinoma. An IR should differentiate fever secondary to hepatic abscess from postablation syndrome. High fever lasting longer than 2 weeks should be alerted. For patients with diabetes, the fasting blood glucose should be controlled under 8.0 mmol/L. For larger lesions, two or more sessions are recommended to prevent the formation of liver abscess. Once abscess occurs, adequate broad-spectrum antibiotic should be used as soon as possible. Then, appropriate medication should be selected according to drug resistance and blood culture. US-guided catheter drainage should be performed if necessary (Fig. 6.1–6.4).


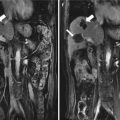
Fig. 6.1
Hepatic abscess in a 42-year-old man with liver metastasis from rectal cancer. (a) Contrast magnetic resonance imaging obtained before ablation showed a moderate enhancement of the nodule in the left lateral segment with the size of 1.6 × 1.2 cm (white arrow). (b) Follow-up competed tomography (CT) showed air-containing abscess in the ablated area (black arrow). (c) Ultrasound (US)-guided percutaneous drainage (white arrow). (d) Magnetic resonance imaging obtained 3 months after drainage showed obvious absorption (white arrow)
6.2.1.4 Hepatic Failure
Liver failure after ablation is not common but often fatal. It mainly occurs in patients with multiple tumors or a Child-Pugh score of B or above. Increased ablation sessions or large volume of liver coagulative necrosis may add such risks in patients with liver cirrhosis [5]. In our latest study, no hepatic failure was recorded. Strict control of indications and cautious management of large or multiple lesions in patients with limited hepatic reserve capacity will be helpful to decrease such risks. Any sign of prolonged hepatic decompensation after ablation should be carefully followed and early aggressively treated.
6.2.1.5 Biliary Complications
Bile Duct Injury
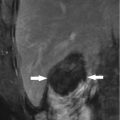
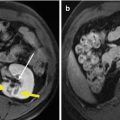
Bile duct injury, including biliary stenosis, cholangitis, biloma, biliary fistula, etc., mainly occurs in patient with tumor adjacent to the hepatic hilum (Fig. 6.2). Excessive heating or mechanical injury secondary to electrode placement may cause damage to the major bile ducts. However, severe bile duct injury is not common. The incidence rate was 0.1–0.7 %. Cooling of the main bile duct with chilled isotonic saline solution and implantation of prophylactic stent have been documented [10, 11], but these techniques are difficult to perform for percutaneous procedure, thus seldomly used. Our latest results showed only one case of biliary stenosis in the primary group and one case of biloma in the metastatic group due to the hepatic helium location and increased number of ablation session. Several tips in our center are as follows: long-duration and low-power microwave emission (45–50 W), real-time temperature monitoring at the periphery of the tumor, and combined ethanol injection will be helpful for minimizing major bile duct injuries. Injury of second branches or above may cause bile duct stricture or even severe obstructive jaundice, which requires percutaneous transhepatic cholangial drainage. For serious bile duct stenosis of the left and right branches, a stent implantation will be necessary (Fig. 6.2).


Fig. 6.2
Biloma in a 62-year-old man with liver metastatic nasopharyngeal carcinoma (3.9 × 4.1 cm in size) (Picture Credits: Liang et al. [2]. Copyright holder: 2009 Radiological Society of North America). (a) Contrast CT obtained 1 month after microwave ablation (MWA) showed biloma represented by an area of fluid-filled cavity (black arrow) and another complete ablated tumor represented by low-density lesion in the right posterior lobe (arrowhead). (b) After subsequent percutaneous drainage (black arrow). (c) CT scan showed resolution of biloma after 3 months of drainage (black arrow)
Gallbladder Injury
The risk of gallbladder (GB) injury increases when there is ablation of a mass adjacent to the GB bed. Inflammatory changes in the GB wall that manifest as minimal wall thickening are common, but GB perforation is very rare due to liquid flow within it that dissipates the heat [18]. Adhesion of GB and its surrounding organs caused by previous surgery is a risk factor for potential GB injury. Cholecystokinin-assisted hydrodissection of the GB fossa has been reported to decrease the risk of such complication [19]. To avoid mechanical damage to the GB, real-time US should be performed to monitor the position of MW electrode. Our experiences [20] showed that real-time temperature monitoring during MWA (<54 °C), with a small amount of assisted ethanol injection, is a key point to minimize the thermal-mediated GB injury. Anti-infection treatment, if necessary, percutaneous US-guided drainage, or cholecystectomy should be performed to these patients with acute cholecystitis or severe edema of the GB after ablation.
6.2.1.6 Gastrointestinal Tract Perforation
Gastrointestinal tract (GIT) perforation mainly occurred in patients with tumors adjacent to the GIT, which is life-threatening, if not properly managed, and may cause septic shock or even death. The incidence rate was 0.1–0.2 %. Fibrotic adhesion between the bowel and the liver due to history of abdominal surgery is the most common cause of such complication. In our experience, colon involvement is relatively more common, because it is relatively fixed with a thin wall and poor blood supply compared with the small intestine or stomach wall [21]. No GIT perforation was observed in our latest study due to the selected puncture path and ablation-assisted techniques. Strict preoperative bowel preparation is one of the important measures of prevent such complications. Artificial ascites is a key point to separate the target tumor from the GIT [22]. Real-time monitoring of the temperature of the marginal tissue proximal to the GIT and adjuvant therapy with small dose of ethanol injection in the vicinity of the marginal tissue of tumor may prevent the GIT from thermal damage and achieve complete necrosis of tumor margin [23]. An IR should carefully perform the ablation procedure and closely follow the clinical symptoms and should carefully assess whether there is a thickening bowel wall adjacent to the target lesions. When perforation does occur, surgical repair should be performed immediately.
6.2.1.7 Portal Vein Thrombosis
PVT mainly occurs in patient with a centrally located tumor in the immediate vicinity of a compressed portal vein. Thrombosis of vessels larger than 4 mm in diameter is uncommon because of the heat-sink effects of blood flow. Extensive heat, slow portal flow caused by liver cirrhosis, or interrupted real-time scan due to gas bubbles after MW irradiation may be responsible for PVT [5, 24]. In our latest study, PVT was founded in only one patient with tumor adjacent to hilar portal vein. Our tips to decrease such complication are as follows: long-duration and low-power (45–50 W) ablation, real-time peritumoral temperature monitoring, additional ablation sessions, and small dose of ethanol injection [25]. Although often asymptomatic, PVT requires great attention as it is occasionally associated with aggravated portal hypertension, worsened liver function or even liver failure, mesenteric vein thrombosis, or small bowel infarction. Once acute PVT happens, fibrinolytic therapy using high dose of urokinase is a reasonable treatment method [26].
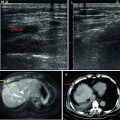
6.2.1.8 Thoracic-Diaphragm Complications
Thoracic-diaphragm complications, including pleural effusion, pneumothorax, hemothorax, emphysema, and diaphragmatic hernia, often occur in patients with tumors located close to the diaphragm [2, 4, 5, 11, 22, 26–31] (Fig. 6.3). Pleural effusion requiring drainage was most common, with the occurrence rate ranging from 0.4 to 1.7 %. The main cause is thermal damage to the diaphragm due to the tumor location adjacent to the diaphragmatic dome. Serious injury may lead to diaphragmatic hernia or perforation. Hemothorax or pneumothorax is infrequent [4, 5]; the possible causes are injuries to the intercostal or diaphragmatic blood vessels secondary to electrode placement. Artificial hydrothorax or ascites may effectively lower the incidence of diaphragm injury, due to both thermal buffering and better visualization [14, 32]. Close observation of the changes of patients’ clinical symptoms is recommended. When chest pain and dyspnea happen, computed tomography (CT) or US will be necessary to determine the absence and amount of liquid. Small to moderate amount of pleural effusion does not require treatment, while the large ones with symptoms of dyspnea should be treated with aspiration or drainage. An IR should actively look for the causes of hemothorax. For progressive bleeding not responsive to conservative treatment, arterial embolization or thoracotomy will be recommended (Fig. 6.3).