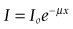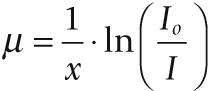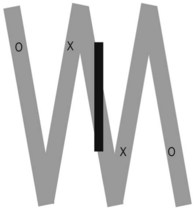Chapter 35 Computed tomography
Introduction
Advantages of CT include
• Axial acquisition of cross-sectional images: with modern isotropic imaging, data can be post processed into multiple planes or rendered volumes, producing 2D or 3D images. Magnetic resonance (MR) is truly multiplanar, as scans are acquired directly in different planes without the need for reconstruction; however, the quality of CT isotropic reconstructions is high.
• Cross-sectional imaging has excellent low-contrast resolution (LCR), which is superior to other imaging methods with the exception of MR, which matches and in some cases exceeds the LCR of CT.
• CT images also show good high-contrast (spatial) resolution and excellent bone detail. MR does not image bone directly owing to the lack of free hydrogen within cortical bone.
• Digital imaging: this enables the manipulation of images, as well as post processing to other planes; the applied algorithm and windows can be adjusted to better visualise specific tissues. The application of filters and digital processing can enhance content, e.g. the use of edge enhancement for looking at bone.
• CT is generally well tolerated by patients, certainly more so than MR, which is less well tolerated owing to noise and claustrophobia. Contraindications for MR due to safety requirements do not apply to CT.
• CT is still more readily available than MRI and radionuclide imaging (RNI), being in situ in the vast majority of DGHs in the UK.
Disadvantages of CT include
• Ionising radiation dose: CT is undeniably an extremely high-dose technique, many examinations being among the highest, if not the highest doses, in use in medical imaging. Multiple examinations may approach the thresholds for deterministic radiation effects.
• Metallic artefacts cause loss of image detail; on many modern scanners this effect is much reduced by software corrections.
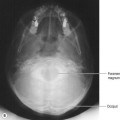
• Soft tissue structures surrounded closely by bone can be difficult to image, e.g. in the posterior fossa, where the soft tissue contrast of MR is superior. This is again a problem largely overcome in the latest generation of scanners.
• Misregistration artefact can be caused by relative movement of the body structures from the acquisition of a single slice to the next, e.g. due to inconsistencies in the patient’s respiratory pattern. If misregistration occurs then the reconstruction will be meaningless, as the same portion of anatomy could be portrayed at different positions in the reconstructed image. With the advent of single breath-hold scanning this is now less of a consideration. However, many centres, when scanning two areas such as the chest and upper abdomen, will overlap the two acquisition blocks to ensure no loss of information due to breathing differences between the two acquisitions. The dose implications of this technique are worthy of consideration.
In some quarters there is an attitude that CT can be undertaken by anybody, including non-radiographically qualified staff such as departmental assistants. It can be argued, however, that, along with every other branch of imaging, CT is operator dependent. Image quality is dependent on factors that should be adjusted for each examination, and more importantly, for each patient. In addition, because of the high dose burden all operators of CT equipment should be trained and skilled in optimising CT examinations;1 indeed, specific additional training requirements are mandatory in some countries, such as the USA;2 unfortunately, the need for requirements such as this can be only too evident.3
Equipment chronology
1874 Sir William Crookes constructs the cathode discharge tube. During his experiments over the next few years he discovers fogging of photographic plates stored near discharge tubes.
1895 Wilhelm Roentgen discovers X-rays while investigating gas discharge using a Crookes’ tube.
1935 Grossman coins the term ‘tomography’ to describe his apparatus for looking at detail in the lungs.4
1951 Godfrey Hounsfield starts work at EMI, initially working on early computers.
1956 Ronald Bracewell uses Fourier transforms to reconstruct solar images. At the same time Alan Cormack starts to work on solving ‘line integrals’.
1958 Korenblyum and colleagues in Ukraine work on obtaining thin-section X-ray images using mathematical reconstructions.
1961 William Oldendorf produces an image of the internal structure of a test object using a rotating object. He was unable to make further progress owing to the lack of available equipment to provide the computation that would have been required.
1963 Cormack publishes a paper on mathematical reconstruction methods.
1965 David Kuhl, one of the pioneers of RNI, produces a transmission image using a radioactive source coupled to a detector.5
1967 Bracewell produces a mathematical solution for reconstruction with fewer errors and artefact than found with Fourier.
1971 The first clinical CT scanner is installed at Atkinson Morley Hospital under the supervision of James Ambrose. The first patient is scanned on 1 October. The first scanners were somewhat crude and took several minutes to produce each slice, which were of fairly poor quality. However, at the time even these crude images were revolutionary, enabling a first non-invasive glimpse at the soft tissue contents of the skull.
1972 Ambrose and Hounsfield discuss the clinical use of CT at the British Institute of Radiology annual conference.6 Clinical images are shown at RSNA.
1973 Hounsfield and Ambrose publish papers describing the design and clinical applications of the CT system.7,8 EMI scanner becomes commercially available.
1974 Hounsfield produces abdominal images with a 20-second acquisition time.
1975 EMI CT 1010 second-generation scanner becomes available, soon to be followed by the CT 5005 – the first EMI body scanner.
1979 Hounsfield and Cormack are awarded the Nobel prize for medicine.
1983 The first 2-second scanner introduced by GE (CT 9800).
1985 Electron beam CT developed.
1989 Siemens introduce spiral (helical) CT, using slip ring technology to enable the tube to rotate continuously without the need to go back to unwind its cables.
1992 Elscint Twin scans two slices simultaneously, which is a return to a method used by the original EMI scanners.
1998 Multislice CT initially incorporating four slices is introduced; GE, Picker, Siemens and Toshiba displayed systems at RSNA. Since then 8-, 16-, 32-, 40-, 64- and 128-slice machines have become available. Sub-second scan times enable body areas to be scanned in a single breath-hold. Advancements have in many cases had to await the development of computer systems robust enough to cope with the huge quantities of data generated, a problem initially encountered by Oldendorf.
2005 Siemens launch dual-energy scanners, opening the way to characterisation of chemical make-up of materials via simultaneous imaging at different kV values.
2007 Toshiba launch Aquilion One, 320-slice, ending the numbers game? Enables single rotation imaging of entire organs due to 16 cm coverage.
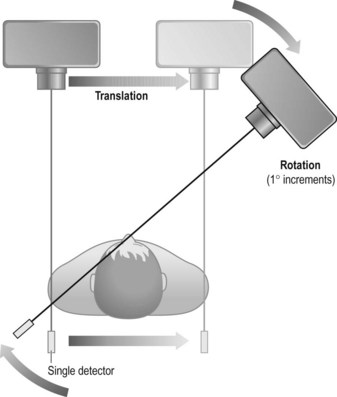
First-generation scanner (Fig. 35.1)
• Advantages: it was the first of its kind and offered the first opportunity for axial imaging of the head
• Disadvantages: mechanically complex, slow scans, which were only practical for scanning the head of patients who could be adequately immobilised using a water bag. The water bag was used to reduce the range of information required, as its density is closer to air than to that of tissue
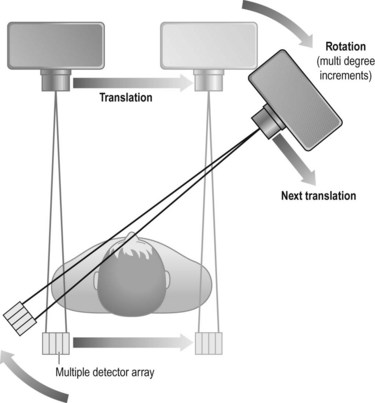
Second-generation scanner (Fig. 35.2)
• Advantages: as several detectors were being used, scanning times were significantly reduced and quality was increased. Typical scan times of the order of 20–80 seconds per slice were achievable. Again, two slices were acquired simultaneously on the EMI 1010 with a fixed slice thickness of 13 mm
• Disadvantages: the maintenance of the translate–rotate movement renders these scanners still mechanically complex
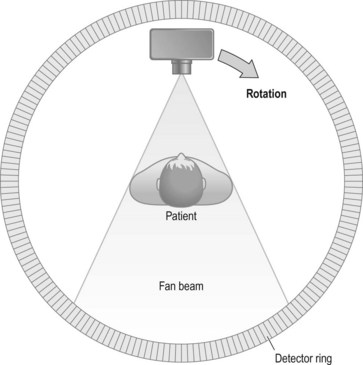
Third-generation scanner (Fig. 35.3)
• Advantages: the greater number of detectors plus the rotation-only movement allows shorter scan times, typically of the order of 2–8 seconds. The width of the fan beam can be adjusted (collimated) to limit the beam to the area under examination. Use of the rotation-only movement renders this type of unit mechanically simpler than its predecessors
• Disadvantages: detectors were expensive, therefore more detectors equals more cost. Also more processing power is required, as more information is gathered at one time. Initially problems were encountered with circular artefacts, but this was overcome by adjusting the detectors
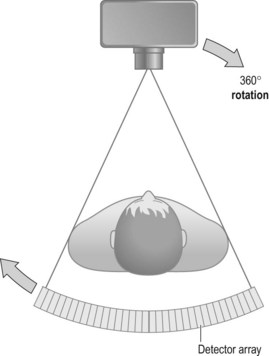
Fourth-generation scanner (Fig. 35.4)
• Advantages: mechanically simpler owing to having fewer moving parts. Scan times reduced and now taking 1–10 seconds
• Disadvantages: the high number of detectors equals high cost. There were also greater calibration difficulties. As the tube is rotating within the detector ring, the detectors are further away from the patient, leading to a greater penumbral effect
Multislice CT
Advantages of multislice include
• Speed of acquisition – sub-second rotation speeds are now the norm
• Compared to single-slice helical, multislice enables the same acquisition in a shorter time, or larger volumes to be scanned in the same time, or thinner slices to be scanned
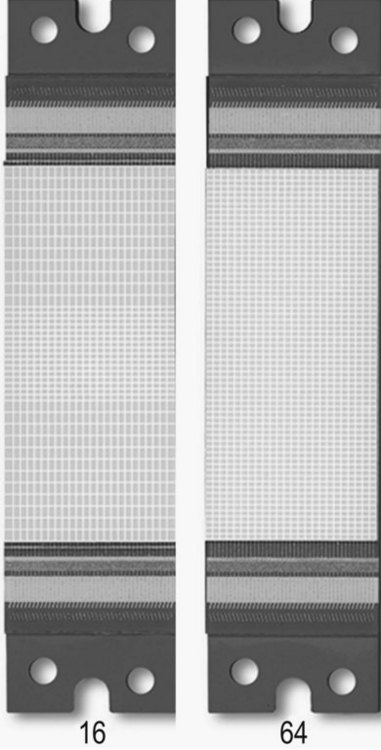
• All manufacturers have sub-millimetre scan capabilities. Toshiba have detectors that are 0.5 mm, matching the pixel size to produce a voxel which is the same size in each dimension: termed isotropic (see Fig. 35.10). Isotropic and near isotropic voxels enhance the 2D reformatting ability of the scanner, enabling high-quality multiplanar reconstructions from an axial data set. 3D reformats produced are also excellent, with none of the problems of possible misregistration and information loss inherent in MR owing to its longer scan times.
Equipment
Detectors
Modern detectors are of the solid state type, mostly using ultrafast ceramic detector elements. An incident beam causes scintillation; the photon produced is then converted to an electrical signal by a photodiode and sent on to the electronics. The detector array is formed by a series of individual elements, as shown in Figure 35.5.
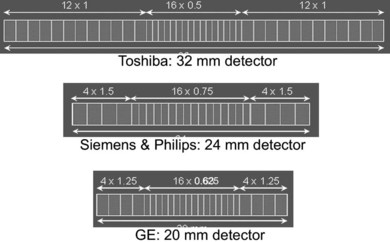
Different manufacturers have differing approaches to the format of detector arrays, with four-slice machines being available as fixed matrix, adaptive or mixed arrays. Each of the major manufacturers has taken a different approach to 16-slice, and as can be seen in Figure 35.6, the choice of array format affects the minimum slice width available, the number of slices available at minimum width, and the range of slice widths available.
Physical principles of scanning
For the energies used in CT the attenuation of the beam is due to:
I is measured by the detectors, Io and x are known, hence µ can be calculated.
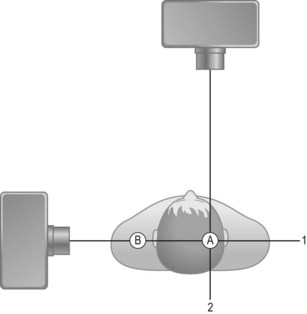
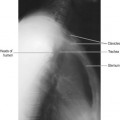
Traditionally a narrow beam was required for accurate localisation of the attenuating tissues. Readings are taken from multiple angles to give a series of values of linear attenuation of the beam along intersecting lines through the patient. For example, in Figure 35.7 a bony object would have the same attenuating effect on ‘beam 1’ whether at position ‘A’ or ‘B’. However, from ‘beam 2’ it is possible to localise the structure to position ‘B’.
The information acquired by the detectors is passed to the computer. Once this data is committed to the computer memory it can be manipulated by the resident software to produce an image which is reconstructed on the screen of the viewing console. Reconstruction takes place via the application of a complex mathematical algorithm to the data obtained, usually a filtered back projection. Consideration of the detail of this mathematical process is beyond the scope of this chapter, but is well described in texts such as Seeram.9
Helical image reconstruction is more complex: because the table is continuously moving only one ray sum lies in the scan plane; the rest of the ‘slice’ information is interpolated from the acquired volume. 360° and 180° interpolations are used. As seen in Figure 35.8, a 360° interpolation requires data from two tube rotations for slice reconstruction. 180° interpolation allows smaller slice widths to be accurately reconstructed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree