All radiologic contrast media available for intravascular use depend on iodine for their radiopacity. Ideally, contrast media should be inert in every respect. But unlike other therapeutic medications, contrast media are used in larger quantities and participate in numerous physiologic and pharmacokinetic interactions after intravenous administration. Their interactions not only affect tissue characteristics but also may have significant effects on patient health.
Contrast Enhancement Principles
The principle of contrast enhancement is based on photoelectric interaction of the iodine atom with x-rays. The binding energy of the inner K shell electron of the iodine atom is called its K-edge and is equal to 33.2 keV. The mean energy of diagnostic radiographs is close to this value. When diagnostic radiographs interact with iodine atoms in the body there is increased absorption of the x-rays by the iodine atoms compared with the surrounding soft tissues. The attenuation of the x-ray beam increases with the concentration of iodine in the tissue because of more K shell interactions. This is the fundamental basis of contrast enhancement. There is a linear relation between iodine concentration and attenuation. Every milligram of iodine in a milliliter of blood or cubic centimeter of tissue elevates the attenuation by 25 Hounsfield units (HU).
Types of Contrast Media
All currently used computed tomography (CT) contrast agents are based on the triiodinated benzene ring. Whereas the iodine atom is responsible for the radiopacity of contrast media, the organic carrier is responsible for its other properties, such as osmolality, tonicity, hydrophilicity, and viscosity. The organic carrier is responsible for most of the adverse effects and has received a lot of attention from researchers. Some patients react to small amounts of contrast media, but most of the adverse effects are mediated by the large osmotic load. Thus, over the past few decades researchers have focused on developing contrast media that minimize the osmotic load after contrast agent administration.
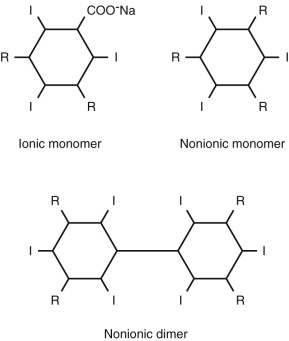
Contrast media are classified as ionic or nonionic and as monomers or dimers. Ionic contrast media dissolve in water to dissociate into an iodinated benzene ring containing an iodized carboxyl group and a cation. Nonionic contrast media are water soluble (hydrophilic) but do not dissociate in solution. Monomers have a single triiodinated benzene ring, whereas dimers have a double benzene ring containing six iodine atoms. Figure 8-1 shows the chemical structure of contrast media molecules.
The most commonly used classification of contrast media is by their osmolality, which is defined as the number of osmotically active particles per kilogram of solvent. This is determined by the size of the contrast media molecule and whether it dissociates in solution. Table 8-1 lists commonly used contrast media.
| Classification | Iodine per Particle Ratio | Osmolality (mOsm/kg) |
|---|---|---|
| High osmolar contrast media | 3 : 2 | 1500-1800 |
| Diatrizoate (Hypaque) | ||
| Iothalamate (Conray) | ||
| Nonionic monomer low-osmolar contrast media | 3 : 1 | 600-700 |
| Iohexol (Omnipaque) | ||
| Iomeprol (Iomeron) | ||
| Iopamidol (Niopam) | ||
| Iopromide (Ultravist) | ||
| Ioversol (Optiray) | ||
| Ioxilan (Oxilan) | ||
| Iobitridol (Xenetix) | ||
| Ionic dimer low-osmolar contrast media | 3 : 1 | 560 |
| Ioxaglate (Hexabrix) | ||
| Nonionic dimer isosmolar contrast media | 6 : 1 | 300 |
| Iodixanol (Visipaque) | ||
| Iotrolan (Isovist) |
High Osmolar Contrast Media
Ionic monomers consisting of a cation (e.g., sodium, meglumine) and an anion (iodine-containing benzoic acid derivative) were developed in the 1950s. These molecules dissociate in solution to give three atoms of iodine for every two particles in solution (iodine atoms to particle in solution ratio of 3 : 2). The osmolality of these agents ranges from 1500 to 1800 mOsm/kg, whereas that of human plasma is 290 mOsm/kg; thus, they are named high osmolar contrast media . Because of the higher incidence of adverse reactions, these agents are infrequently used today.
Low Osmolar Contrast Media
Nonionic monomers are widely used today and have better solubility and lower toxicity compared with ionic monomers. Nonionic monomers consist of a benzoic acid derivative containing three iodine atoms per molecule but do not contain an ionizing carboxyl group (iodine atoms to particle in solution ratio of 3 : 1). These particles do not dissociate in solution. Their osmolality is 600 to 700 mOsm/kg (double that of human plasma). Other than eliminating ionicity and reducing osmolality, newer low osmolar contrast media also have an increased number of, and more evenly distributed, hydroxyl groups. This improves their hydrophilicity by restricting access to the lipophilic areas of the molecule, decreasing affinity for cell membrane and plasma proteins and improving tolerance.
Dimeric ionic contrast media contain six atoms of iodine per molecule. They dissociate in solution into an anion (six-iodine atom double-benzene ring) and a cation, yielding a ratio of iodine atoms to particle in solution of 6 : 2 or 3 : 1. The osmolality of ionic dimers is only slightly lower than that of nonionic monomers.
Isosmolar Contrast Media
Nonionic dimers have been more recently developed. These molecules consist of a double-benzene ring with six iodine atoms. Because they do not dissociate in solution, they have a ratio of iodine atoms to particle in solution of 6 : 1 and are isosmolar to plasma. The larger molecule size in isosmolar contrast media results in higher viscosity. Nonionic dimers have generally been shown to be less nephrotoxic compared with other compounds, but this is not established.
Types of Contrast Media
All currently used computed tomography (CT) contrast agents are based on the triiodinated benzene ring. Whereas the iodine atom is responsible for the radiopacity of contrast media, the organic carrier is responsible for its other properties, such as osmolality, tonicity, hydrophilicity, and viscosity. The organic carrier is responsible for most of the adverse effects and has received a lot of attention from researchers. Some patients react to small amounts of contrast media, but most of the adverse effects are mediated by the large osmotic load. Thus, over the past few decades researchers have focused on developing contrast media that minimize the osmotic load after contrast agent administration.
Contrast media are classified as ionic or nonionic and as monomers or dimers. Ionic contrast media dissolve in water to dissociate into an iodinated benzene ring containing an iodized carboxyl group and a cation. Nonionic contrast media are water soluble (hydrophilic) but do not dissociate in solution. Monomers have a single triiodinated benzene ring, whereas dimers have a double benzene ring containing six iodine atoms. Figure 8-1 shows the chemical structure of contrast media molecules.

The most commonly used classification of contrast media is by their osmolality, which is defined as the number of osmotically active particles per kilogram of solvent. This is determined by the size of the contrast media molecule and whether it dissociates in solution. Table 8-1 lists commonly used contrast media.
| Classification | Iodine per Particle Ratio | Osmolality (mOsm/kg) |
|---|---|---|
| High osmolar contrast media | 3 : 2 | 1500-1800 |
| Diatrizoate (Hypaque) | ||
| Iothalamate (Conray) | ||
| Nonionic monomer low-osmolar contrast media | 3 : 1 | 600-700 |
| Iohexol (Omnipaque) | ||
| Iomeprol (Iomeron) | ||
| Iopamidol (Niopam) | ||
| Iopromide (Ultravist) | ||
| Ioversol (Optiray) | ||
| Ioxilan (Oxilan) | ||
| Iobitridol (Xenetix) | ||
| Ionic dimer low-osmolar contrast media | 3 : 1 | 560 |
| Ioxaglate (Hexabrix) | ||
| Nonionic dimer isosmolar contrast media | 6 : 1 | 300 |
| Iodixanol (Visipaque) | ||
| Iotrolan (Isovist) |
High Osmolar Contrast Media
Ionic monomers consisting of a cation (e.g., sodium, meglumine) and an anion (iodine-containing benzoic acid derivative) were developed in the 1950s. These molecules dissociate in solution to give three atoms of iodine for every two particles in solution (iodine atoms to particle in solution ratio of 3 : 2). The osmolality of these agents ranges from 1500 to 1800 mOsm/kg, whereas that of human plasma is 290 mOsm/kg; thus, they are named high osmolar contrast media . Because of the higher incidence of adverse reactions, these agents are infrequently used today.
Low Osmolar Contrast Media
Nonionic monomers are widely used today and have better solubility and lower toxicity compared with ionic monomers. Nonionic monomers consist of a benzoic acid derivative containing three iodine atoms per molecule but do not contain an ionizing carboxyl group (iodine atoms to particle in solution ratio of 3 : 1). These particles do not dissociate in solution. Their osmolality is 600 to 700 mOsm/kg (double that of human plasma). Other than eliminating ionicity and reducing osmolality, newer low osmolar contrast media also have an increased number of, and more evenly distributed, hydroxyl groups. This improves their hydrophilicity by restricting access to the lipophilic areas of the molecule, decreasing affinity for cell membrane and plasma proteins and improving tolerance.
Dimeric ionic contrast media contain six atoms of iodine per molecule. They dissociate in solution into an anion (six-iodine atom double-benzene ring) and a cation, yielding a ratio of iodine atoms to particle in solution of 6 : 2 or 3 : 1. The osmolality of ionic dimers is only slightly lower than that of nonionic monomers.
Isosmolar Contrast Media
Nonionic dimers have been more recently developed. These molecules consist of a double-benzene ring with six iodine atoms. Because they do not dissociate in solution, they have a ratio of iodine atoms to particle in solution of 6 : 1 and are isosmolar to plasma. The larger molecule size in isosmolar contrast media results in higher viscosity. Nonionic dimers have generally been shown to be less nephrotoxic compared with other compounds, but this is not established.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree