Etiology
Benign prostatic hyperplasia (BPH) is characterized by the increased volume of prostatic stroma and glandular epithelial cells involving the transitional zone and periurethral region of the prostate. Hormones such as androgens (testosterone and dihydrotestosterone [DHT]) and estrogens are thought to play a central role in proliferation of glandular epithelial elements. The neural, endocrine, and immune systems are also implicated in the prostatic tissue remodeling process.
Prostate tissue remodeling in the transitional zone is characterized by (1) hypertrophic basal cells, (2) calcification and inflammation, (3) lymphocytic infiltration, (4) increased radical oxygen species production, (5) increased basic fibroblast growth factor and transforming growth factor-beta 1 production, (6) altered autonomic innervation, and (7) altered neuroendocrine cell function with release of neuroendocrine peptides. Smooth muscle hypertrophy resulting in increased tone in the prostate, the prostate capsule, and the bladder neck leads to increased intrinsic prostatic urethral resistance, contributing to the sequelae of BPH. These factors—glandular enlargement and increased smooth muscle tone—are targeted with medical therapy.
Prevalence and Epidemiology
The prostate gland gradually increases in size as men age. It is estimated that nearly 80% of men will develop BPH and as many as 30% will receive treatment for this condition during their lifetime. One in four men older than the 40 years of age will develop symptoms of BPH. More than 50% of men in the United States between the ages of 60 and 70 and as many as 80% between the ages of 70 and 90 have symptoms of BPH.
Prevalence and Epidemiology
The prostate gland gradually increases in size as men age. It is estimated that nearly 80% of men will develop BPH and as many as 30% will receive treatment for this condition during their lifetime. One in four men older than the 40 years of age will develop symptoms of BPH. More than 50% of men in the United States between the ages of 60 and 70 and as many as 80% between the ages of 70 and 90 have symptoms of BPH.
Clinical Presentation
The presenting symptoms of BPH are classified as obstructive or irritative. Obstructive symptoms include hesitancy, intermittency, incomplete voiding, weak urinary stream, and straining. Irritative symptoms include frequency of urination, nocturia, and urgency. Digital rectal examination (DRE) is commonly performed in assessing BPH. DRE tends to underestimate prostate size because much of the hyperplasia may occur away from the examining finger, which primarily feels the posterior prostate.
The symptoms of BPH may be evaluated using the American Urological Association (AUA) Symptom Index (also known as the International Prostate Symptom Score [IPSS]). This scoring system was designed to assess the severity of BPH. It consists of seven symptoms: frequency, nocturia, weak urinary stream, hesitancy, intermittence, incomplete emptying, and urgency, each of which is scored on a scale of 0 (not present) to 5 (almost always present). Symptoms are classified as mild (total score, 0 to 7), moderate (total score, 8 to 19), and severe (total score, 20 to 35).
Prostate-specific antigen (PSA) may be elevated in both BPH and prostate cancer. BPH can increase PSA levels to two to three times normal owing to increased organ volume and/or inflammation. PSA density, PSA free percentage, and, ultimately, transrectal ultrasound (TRUS)-guided biopsy may be required to differentiate between the two pathologic processes. BPH is not a premalignant lesion, and having BPH does not seem to increase the probability of developing prostate cancer.
However, BPH may cause significant problems if left untreated. Patients may present with complications such as cystitis, recurrent urinary tract infections (UTIs), bladder calculi, and acute or chronic urinary retention. Some patients who have chronic urinary retention may eventually progress to renal failure. Early detection of BPH lowers the risk for bladder and renal damage caused by chronic urinary tract obstruction.
Normal Anatomy
The prostate is a cone-shaped exocrine gland located inferior to the bladder and anterior to the rectum. It surrounds the uppermost segment of the urethra and is enveloped by an incomplete fibromuscular capsule. The gland contains a base superiorly, a mid-gland, and an apex inferiorly. It can be divided into lobes, which include the anterior lobe, posterior lobe, lateral lobes, and median lobe. The posterior lobe is palpated on DRE, and the median lobe can enlarge at the midline of the prostatic base and protrude into the base of the bladder. Important neurovascular structures lie within the pericapsular fat anterior to the apex (anterior periprostatic plexus) and posterolaterally (neurovascular bundles). The neurovascular bundles innervate the corpus cavernosum and are critical for normal erectile function.
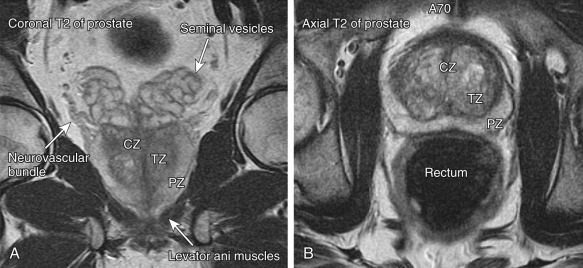
The anatomic zonal architecture of the prostate gland is divided into three main regions: peripheral zone, central gland, and anterior fibromuscular stroma ( Figures 72-1 and 72-2 ). The central gland is composed of the transitional zone and central zone. The peripheral zone occupies the posterolateral compartment of the prostate gland. It occupies the majority of the prostate volume in young men and is the origin of up to 70% of adenocarcinomas. The transitional zone surrounds the prostatic urethra proximal to the verumontanum. It accounts for only 5% to 10% of prostate volume in young men but is the zone responsible for prostatic enlargement in the setting of BPH. Up to 20% of prostate cancers occur in the transitional zone. The central zone surrounds the ejaculatory ducts and makes up approximately 25% of the prostate in young men. Only 1% to 5% of prostate cancers arise in the central zone. Carcinoma is similarly uncommon in the anterior fibromuscular stroma.

The architecture of the prostate gland changes with age. From the mid-20s, the prostate begins to gradually enlarge. The central zone atrophies, and the transitional zone enlarges secondary to BPH, with subsequent compression of the urethra. Although large prostate glands are more likely to cause symptoms of BPH, obstructive symptoms correlate poorly with gland size.
Pathophysiology
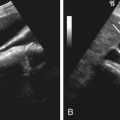
The pathophysiologic processes of BPH are poorly understood. BPH is thought to result from both static (androgen-induced hyperplasia) and dynamic (increased smooth muscle tone) processes. Prostate gland hyperplasia and increased smooth muscle tone cause gradual compression of the urethra, interrupting the normal flow of urine ( Figure 72-3 ). The bladder compensates by increasing micturating pressures to overcome the obstruction leading to detrusor muscle hypertrophy. This, in turn, leads to more frequent bladder contractions, more frequent micturition, and symptoms of urinary hesitancy and frequent urination. Detrusor muscle thickening also causes bladder wall trabeculation and formation of diverticula ( Figure 72-4 ).


The bladder wall cannot maintain high micturating pressures and, in time, decompensates and weakens. It starts to accommodate high volumes of urine and fails to empty normally. This results in incomplete micturition and high residual urine volumes, which predispose to cystitis and bladder stone formation. There may be gradual development of hydroureter and hydronephrosis, which can ultimately lead to renal failure ( Figure 72-5 ).

Imaging
Imaging of the prostate and the urinary tract is not recommended in the routine evaluation of men with prostatism unless there are symptoms suggesting complications of BPH (e.g., recurrent UTI), findings suggesting another diagnosis (e.g., hematuria, marked prostatic asymmetry on DRE), or a history of previous urologic surgery.
TRUS and MRI provide detailed information about the internal structure of the prostate and pathologic prostate enlargement. These modalities are more accurate in estimating prostate volume than DRE and transabdominal ultrasound.
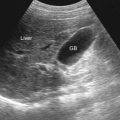
Transabdominal ultrasound is the preferred method of determining postvoid residual volume. The significance of an increased postvoid residual volume is that it indicates bladder dysfunction and is associated with a less favorable response to treatment or treatment failure. Measuring postvoid volume is recommended in those with symptomatic BPH by the AUA and the European Urological Association (EUA).
Computed Tomography
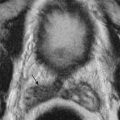
CT can demonstrate prostate enlargement and define the gland’s relationship to other pelvic organs ( Figures 72-6 and 72-7 ). However, it requires ionizing radiation and does not accurately define prostatic zonal anatomy. Thus, the value of CT in the diagnosis and management of patients with BPH is limited.