Computed tomography (CT) is one of the main diagnostic methods for assessing both acute gastrointestinal bleeding (GIB) and bowel ischemia due to its widespread availability, excellent spatial resolution, and high accuracy. While endoscopy is the preferred diagnostic tool for workup of upper GIB, CT is used in select instances as a complementary modality or when endoscopy is impractical. For lower GIB, CT is one of the first-line imaging tools. Mesenteric ischemia is primarily diagnosed with CT, which can exquisitely assess the vasculature and demonstrate bowel findings of ischemia or infarction.
Key points
- •
Computed tomography (CT) is highly accurate for diagnosis of gastrointestinal bleeding and bowel ischemia.
- •
Proper CT technique is essential for an accurate diagnosis.
- •
Knowledge of the differential diagnosis and pathophysiology of acute gastrointestinal bleeding and bowel ischemia are essential for precise diagnosis.
Background
Acute gastrointestinal bleeding (GIB) is a serious medical issue, with potentially significant morbidity and mortality, particularly if diagnosis and treatment are delayed. Each year, GIB accounts for approximately 750,000 emergency department visits, 500,000 inpatient admissions, and 2.2 million hospital days in the United States. Furthermore, mortality from acute GIB is 10% to 14%, but it can rise to 40% in the setting of massive bleeding with hemodynamic instability or substantial blood transfusions. The burden on the health care system is substantial, costing up to US$5 billion per year. Rapid and accurate diagnosis is vital to maximize patient outcomes.
Differential diagnosis
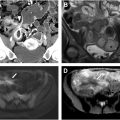
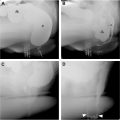
The differential diagnosis for acute GIB is very broad, with at least 2 dozen potential causes. Despite a bewildering list of potential etiologies, approximately 75% of cases of upper GIB are due to peptic ulcer disease in noncirrhotic patients ( Fig. 1 A–D ). Other less common causes of upper GIB include malignancy, Mallory–Weiss tears, and vascular lesions ( Fig. 2 A–D ). Variceal bleeding may account for 50% to 60% of upper GIB in cirrhotic patients with portal hypertension.


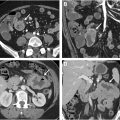
Similarly, in the lower tract, only a small number of entities account for the bulk of acute GIB cases. The most common causes of small bowel bleeding include inflammatory bowel disease in young adults, and angioectasias in the elderly. Other less common possibilities include malignancy ( Fig. 3 A, B ) and non-steroidal anti-inflammatory drugs (NSAID) enteropathy. Diverticulosis is the most common etiology for acute bleeding arising from the colon. Other causes include colorectal carcinoma, ischemia, and hemorrhoids. Importantly, 15% of lower GIB cases may originate from an upper gastrointestinal tract source.

Definitions
To narrow the differential diagnosis, it is important to understand several important definitions. The upper gastrointestinal tract includes the esophagus, stomach, and duodenum to the ligament of Treitz, where approximately 75% of acute GIB cases originate. , The remainder of cases arise from the lower gastrointestinal tract, which can be subdivided into small bowel and colon owing to differences in management, workup, and differential diagnosis.
In addition, several clinical descriptors can help to narrow the differential diagnosis. Overt GIB is clinically obvious, with symptoms such as hematemesis and hematochezia. Occult GIB is less apparent and may only present with iron deficiency anemia or positive fecal occult blood testing. Obscure GIB is reserved for cases in which no clear etiology has been discovered, despite negative imaging and endoscopy.
Diagnostic approach
Once the suspected site of GIB has been narrowed to either the upper or the lower gastrointestinal tract based on clinical presentation, diagnosis can be made using a variety of tools, including endoscopic methods and imaging. It is critical for the radiologist to understand the various options and algorithms in the diagnosis of acute GIB.
Computed tomography imaging
Computed tomography (CT) angiography (CTA) has become increasingly used for detecting acute GIB in recent years due to improvements in spatial resolution and widespread availability. CTA is sensitive for detecting bleeding rates between 0.1 and 0.3 mL/min, which is superior to angiography. , Typically, CT angiography is performed with an initial noncontrast acquisition, which is crucial for identifying hyperdense materials such as ingested pills or debris, which can otherwise be mistaken for sites of active bleeding on subsequent postcontrast phases. Noncontrast imaging can also identify dense intraluminal hemorrhage (sentinel clot), where attention on contrast-enhanced phases can be focused. Subsequently, a late arterial phase (25–35 seconds after contrast bolus initiation), and at least one additional phase: portal venous phase (60–70 seconds) and/or delayed phase (90 seconds) are acquired. The arterial phase identifies active extravasation and provides a roadmap of the visceral arteries for treatment planning. The arterial phase is also used to generate postprocessed 3 dimensional images that may facilitate diagnosis and treatment planning. The portal venous and delayed phases increase sensitivity of identifying active bleeding that may be subtle, slow, or venous. Furthermore, the portal venous phase shows peak solid organ and bowel wall enhancement and, thus, may also identify end-organ ischemia due to acute blood loss anemia. Oral contrast should not be administered as this may obscure or dilute contrast extravasation (if present), prolong examination time in hemodynamically unstable patients, and complicate treatment if patients are meant to be nil per os (NPO) prior to anticipated treatment.
Typical findings of acute GIB include a sentinel clot sign on noncontrast imaging, followed by a focus of contrast extravasation on arterial phase images, which subsequently grows and becomes more amorphous on venous and/or delayed phase images.
Dual-energy CT, although described by Hounsfield in the initial publications on CT in 1973, has only become feasible and widely available in recent years due to hardware developments. By acquiring data at low-energy and high-energy spectra, dual-energy CT allows for differentiation among various materials based on their attenuation characteristics at the 2 energy levels. Iodine density can be amplified by using monoenergetic low kiloelectronvolt reconstructions near the k-edge of iodine, which can significantly increase the conspicuity of iodinated contrast and sites of active hemorrhage. Liu and colleagues demonstrated an 89% GIB detection rate using dual-energy CT compared to 66% using single-energy CT in a phantom model. In addition, dual-energy CT can decrease overall radiation dose if true noncontrast acquisitions are replaced by virtual noncontrast images. Furthermore, iodine density reconstructions allow for quantification of iodine in a region of interest, and color overlays may further aid in the detection of GIB. Photon counting CT is a technical advancement over conventional and dual-energy CT and has the potential to further improve the detection of iodinated contrast; however, availability remains limited.
CT enterography (CTE) is a variant in which up to 2 L of neutral nonabsorbable oral contrast is ingested before imaging to achieve luminal distention and improved visualization of the intestinal walls. CTE is performed with intravenous contrast in the arterial and venous phases if a vascular lesion is suspected, usually in an older patient population. Alternatively, imaging may be limited to a single venous phase if inflammatory bowel disease is suspected, which can be helpful to minimize radiation in the younger patient population that is typically affected. , CTE can be particularly valuable in the setting of occult GIB, which commonly arises from a small bowel source. A major limitation is that oral contrast may not always be well tolerated by all patients, and the delay caused by contrast preparation may limit the role of CTE in the acute setting. CT imaging technique is summarized in Table 1 .
| Phase | Timing (Seconds) | CTA | Single Phase CTE | Multiphase CTE | Utility |
|---|---|---|---|---|---|
| Noncontrast | N/A | Mandatory | N/A | N/A | — |
| Late arterial | 25–35 | Mandatory | N/A | Mandatory |
|
| Enteric | 50 | N/A | Mandatory | One of these is mandatory |
|
| Portal venous | 60–70 | One of these is mandatory | N/A |
| |
| Delayed | 90 | N/A | N/A |
|
Other imaging modalities
Angiography is an established method for diagnosis of acute GIB and is accurate for the detection of bleeding rates as low as 0.5 to 1 mL/min. Angiography has a key advantage of treatment with a variety of embolization techniques after the site of bleeding is found. However, angiography may not always be immediately available and may be negative when intermittent bleeding is present. In addition, sedation is required, and there is a small risk of complications.
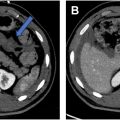
Scintigraphy is a valuable technique for imaging GIB as low as 0.05 to 0.10 mL/min, which is more sensitive than any other imaging modality. Radionuclide-labeled red blood cells are administered intravenously and subsequently may be imaged for up to 24 hours, which is a major advantage in patients with intermittent bleeding, which can be a pitfall with CT angiography ( Fig. 4 A–D ). Furthermore, the lack of iodinated contrast is a significant advantage of scintigraphy in patients with contrast allergies. Drawbacks include relatively poor spatial resolution, long scan times, and limited availability in the acute setting. Despite superior sensitivity for detecting subtle GIB compared to other imaging modalities, adoption has steadily declined in recent years due to these limitations, with increasing usage of CT.

Endoscopy
Endoscopic techniques include upper endoscopy (esophagogastroduodenoscopy), video capsule endoscopy (VCE), and colonoscopy. All are highly effective methods for diagnosis, and upper and lower endoscopies may also be used for treatment of bleeding. Upper endoscopy is up to 98% sensitive and 100% specific for the diagnosis of acute GIB. , Colonoscopy has a diagnostic yield between 74% and 100%. VCE can be particularly useful for the diagnosis of small bowel bleeding after upper and lower endoscopies are negative.
Though highly accurate for diagnosis, the endoscopic methods have some limitations and drawbacks. For example, upper endoscopy may not be feasible in patients with complex postsurgical anatomy such as a Roux-en-Y gastric bypass, in which the gastric remnant may not be easily accessible. VCE may be contraindicated in patients with known or suspected strictures due to the risk of capsule retention. Colonoscopy has the disadvantage of lengthy bowel preparation times, which may limit its utility in the acute setting. Both upper and lower endoscopies require sedation, which may not always be feasible.
Workup of nonvariceal upper gastrointestinal bleeding
Typically, patients presenting with nonvariceal upper GIB are worked up with upper endoscopy, which can identify the source in 95% of cases. A major benefit is the potential for treatment once the site of bleeding has been identified. Imaging is usually not the first method for diagnosis of upper GIB, but may be valuable in certain scenarios, as defined by the American College of Radiology appropriateness criteria. In the first scenario, endoscopy identifies the source of bleeding but fails to achieve hemostasis, in which case angiography with the intent to treat is recommended. In the second scenario, endoscopy confirms hemorrhage but cannot control or accurately pinpoint the source. Angiography is typically recommended as the next study, with the potential benefit of treatment. Alternatively, CTA can be performed to localize the source of hemorrhage. In the third scenario, endoscopy is negative despite clinically apparent upper GIB, which may suggest intermittent bleeding. Angiography and CT are recommended as the next steps. In the fourth scenario, upper endoscopy is contraindicated, typically in the setting of altered surgical anatomy (eg, Roux-en-Y gastric bypass). Angiography and CT are the preferred imaging modalities.
Lower gastrointestinal bleeding
Unlike upper GIB, endoscopy is not always the preferred first-line diagnostic examination. Imaging plays a complementary role to colonoscopy in a variety of settings. The American College of Radiology defines 5 scenarios in which imaging is indicated. In the first setting, lower GIB is clinically apparent, and the patient is hemodynamically stable. CT angiography, colonoscopy, and scintigraphy are recommended, but CT is often the preferred diagnostic study due to high accuracy and availability. In the second scenario, lower GIB is clinically obvious, and the patient is hemodynamically unstable and/or has received over 5 units of transfused blood within 24 hours. In this case, angiography is the next best step, but CT may be performed very quickly and may help to pinpoint the source of bleeding in anticipation of angiography. In the third scenario, colonoscopy has localized the bleed but fails to treat the cause. Angiography with the intent to treat is then indicated. Conversely in the fourth scenario, angiography identifies the source but fails to successfully treat the lesion, in which case colonoscopy is recommended. In the fifth scenario, the patient is hemodynamically stable, and upper and lower endoscopies are negative. This usually narrows the site of bleeding to the small bowel, and therefore, capsule endoscopy and CTE are the next best steps.
Background of mesenteric ischemia
Mesenteric ischemia (MI) occurs when blood flow is unable to meet the metabolic demand of the bowel. Ischemia is categorized into acute and chronic presentations, with the acute form representing roughly 95% of cases. , Despite the acute form being more common, it is still relatively rare being responsible for roughly 1 in 1000 hospital admissions. Ischemia can be difficult to diagnose as the presenting symptoms are often variable and nonspecific; a patient’s presenting symptoms may mimic more common pathology such as acute pancreatitis or peptic ulcer disease. High clinical suspicion and obtaining early CT imaging are critical for making the diagnosis as the mortality rate can be as high as 50% to 59% for acute MI (AMI).
Computed tomography assessment of mesenteric ischemia
The American College of Radiology Appropriateness Criteria ranks computed tomography angiography (CTA) of the abdomen and pelvis as “Usually Appropriate” for the initial imaging of both acute and chronic MI. CTA has been shown to be very accurate with reported sensitivity of 64% to 96% and specificity of 92% to 100%. Due to its performance, CTA is considered the first-line imaging modality for the assessment of these patients. CTA also allows for the identification of nonvascular causes of the patient’s symptoms in greater than 60% of cases.
A CTA protocol for the assessment of AMI is summarized in Table 2 . A noncontrast series allows for the detection of mural hyperattenuation, which can represent intramural hemorrhage or atherosclerotic calcification, and serves as a baseline for the evaluation of mural enhancement. , Postcontrast imaging should include both arterial and portal venous acquisitions to facilitate the evaluation of arterial and venous patency. Arterial phase image acquisition can be timed at 20 to 25 seconds after injection or bolus triggered along the descending thoracic aorta. , Venous phase imaging 60 seconds after injection has traditionally been used, but recent practice has moved toward a 90 second delay to ensure homogenous enhancement of the mesenteric veins. Sufficiently high iodinated contrast volume (2 mL/kg with a concentration of 350 I/mL) and injection rate (greater than 4 mL/s) are advised to optimize opacification of the arterial structures. Oral contrast agents are not used in acute presentations due to the time required for administration.